Abstract
The mechanisms of Hebbian synaptic plasticity have been widely hypothesized to play a role in the activity-dependent development of neural circuits. However, these mechanisms are inherently unstable and would lead to the runaway excitation or depression of circuits if left unchecked. In the last decade, a number of elegant studies have demonstrated that homeostatic plasticity mechanisms exist to stabilize neural networks and maintain the constancy of neuronal output in response to changes in activity levels. These include synaptic scaling, sliding threshold models of synaptic plasticity, dynamic regulation of the number and strength of synapses, and bidirectional control of intrinsic excitability. Recently, we showed that the total synaptic input onto individual neurons of the mouse superior colliculus is preserved regardless of the size of their visual receptive fields, a phenomenon we term ‘response homeostasis’. Here, we argue that regulating the capacity for synaptic plasticity and controlling the number and strength of retinocollicular inputs can preserve collicular neuron output, and we present evidence that changes in intrinsic excitability are not associated with response homeostasis. We also review findings from a number of different mutant mice and discuss whether and how different cellular mechanisms may underlie response homeostasis. Combined with other studies, our work reveals an important role for homeostatic mechanisms in regulating functional connectivity during the construction of receptive fields and the refinement of topographic maps.
Neuronal activity is thought to play an instructive role in regulating the functional connectivity of neural circuits during development. These changes are thought to utilize the Hebbian, correlation based mechanisms of long-term potentiation (LTP) and long-term depression (LTD). These Hebbian changes, however, are inherently unstable and can lead to the runaway excitation or depression of a subset of synapses when left unchecked. For example, if LTP is based on the ability of a presynaptic neuron to fire a postsynaptic neuron effectively, then the resulting potentiation will subsequently increase the capacity of that connection to induce firing again in the future, thereby resulting in even greater LTP. This ongoing cycle can eventually cause a destabilizing effect on the circuit (Turrigiano & Nelson, 2004). For this reason, Hebb-based models for experience-dependent development and plasticity of neural circuits typically require some type of homeostatic rules to constrain synaptic strength within certain physiological limits.
In the last decade, a number of studies have investigated homeostatic mechanisms that can permit selective changes at appropriate synapses without degrading the function of the entire neural circuit (Burrone & Murthy, 2003; Turrigiano & Nelson, 2004). In cortical and hippocampal cultures, for example, individual neurons dynamically adjust the strength of all their excitatory synapses during different levels of input activity in order to maintain a target firing rate. In response to a global reduction in afferent activity, excitatory synapses will increase their strength, whereas a global increase in activity will induce an overall reduction in synaptic strength (O'Brien et al. 1998; Turrigiano et al. 1998; Burrone et al. 2002).
One of the mechanisms that may mediate this type of homeostasis is termed ‘synaptic scaling’. In synaptic scaling, the distribution of amplitudes of excitatory currents at all the synapses onto a neuron (the miniature excitatory postsynaptic current; mEPSC) increases in response to reduced activity, and decreases in response to increased activity (Turrigiano et al. 1998). These distributions are scaled up or down in a proportional manner, thereby preserving the relative differences in synaptic weights among all the synapses onto any given neuron (Turrigiano, 1999). Interestingly, synaptic scaling has been observed in the developing visual system in response to changes in sensory input. Depriving one eye of visual input for 2 days (monocular deprivation) results in an increase in the strength of individual synapses onto pyramidal neurons in the rodent visual cortex (Desai et al. 2002). These changes are consistent with the observations of synaptic scaling described in culture, and are reversed with subsequent visual experiences (Desai et al. 2002).
In addition to synaptic scaling, a number of other mechanisms exist that could constrain the output of individual neurons. For example, the threshold for Hebbian LTP and LTD could be changed in order to promote stability and maintain synaptic input around a set point. The same results could also be achieved via the reciprocal regulation of the number and strength of synaptic inputs. Finally, the firing rate of neurons could also be kept constant by adjusting the voltage-dependent active conductances that control intrinsic excitability.
Response homeostasis in the superior colliculus
To examine whether such mechanisms play a role during the refinement of sensory maps and the construction of receptive fields, we have used the mouse retinocollicular system as a model. In this pathway, retinal ganglion cell (RGC) projections to the superficial layers of the superior colliculus (SC) form a precise point-to-point map of visual space. The initial, coarse targeting of RGC axons to their targets is guided by molecular cues expressed in RGCs and the CNS (McLaughlin & O'Leary, 2005). The subsequent refinement of this crude map occurs via a process of activity-dependent competition that requires spontaneous retinal waves (Grubb et al. 2003; McLaughlin et al. 2003; Chandrasekaran et al. 2005). These waves consist of bursts of action potentials that sweep across the retina to produce highly correlated activity among neighbouring RGCs, and are therefore hypothesized to play an instructive role in map refinement by regulating Hebbian competition among retinocollicular synapses (Crair, 1999; Torborg & Feller, 2005; Shah & Crair, 2008).
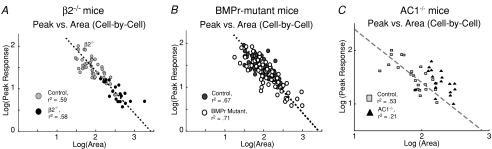
Using various strains of mutant mice with specific mapping defects in the superior colliculus, we have used in vivo recording techniques to test the hypothesis that homeostatic mechanisms exist to preserve the response of individual SC neurons within a given range, a phenomenon we term ‘response homeostasis’ (Chandrasekaran et al. 2005, 2007). In mice that lack the β2 subunit of the nicotinic acetylcholine receptor (β2−/− mice), spontaneous retinal waves are disturbed during the first postnatal week (Bansal et al. 2000). As a result of disrupting this instructive signal, anatomical refinement of the retinotopic map in the superior colliculus (SC) is perturbed in β2−/− animals (McLaughlin et al. 2003; Chandrasekaran et al. 2005). Consistent with the anatomical phenotype, RF areas are, on average, much larger in β2−/− mice. Interestingly, the peak visual response to small stimuli is also much weaker, and the decrease in peak response compensates for the increase in RF area such that the total visual response (defined as the sum of responses across the entire RF) remained constant between genotypes (Chandrasekaran et al. 2007). Because the product of RF area and peak response is proportional to the total response, a plot of the logarithm of these measures should result in a linear relationship of slope −1 if the total response is constant across individual neurons. Figure 1A depicts such a plot, and indeed a line of slope −1 is a good fit for control as well as β2−/− neurons (Control: r2= 0.59; β2−/−r2= 0.52; combined r2= 0.79). The existence of this relationship on a cell-by-cell basis suggests that there is a cellular mechanism in place that regulates neuronal response regardless of receptive field size, both in WT and β2−/− mice.
Figure 1. Response homeostasis is maintained on a cell-by-cell basis in vivo in β2−/− and BMPr-mutant mice but not AC1−/− mice.
All graphs depict scatter plots of receptive field (RF) peak response versus RF area in the log domain on a cell-by-cell basis. A, response homeostasis in β2−/− SC neurons (from Chandrasekaran et al. 2007, reproduced with persmission from the Society for Neuroscience). Both control and β2−/− neuron responses are fitted well by a line of slope −1, demonstrating that, in each cell, RF area and response are precisely coordinated (control as grey circles; r2= 0.59; β2−/− as black circles; r2= 0.58). B, response homeostasis in BMPr mutants (from Chandrasekaran et al. submitted). Plot of RF peak response versus RF area from littermate control (r2= 0.67) and BMPr mutant cells (r2= 0.7) are well fitted by a line of slope −1, demonstrating that the changes in RF shape do not prevent neurons in the SC from maintaining response homeostasis. C, response homeostasis is disrupted in AC1−/− SC neurons (from Shah et al. submitted). Control neuron responses are fitted well by a line of slope −1, demonstrating that, in each cell, RF area and response are precisely coordinated (control as grey squares; r2= 0.53). In AC1−/− neurons, however, such a relationship does not hold, and responses are poorly fitted by a line of slope −1 (AC1−/− as black triangles, r2= 0.21).
We also examined whether response homeostasis persists when the molecular mechanisms that mediated axon guidance and branching of retinal ganglion cell projections to the superior colliculus are disrupted (Chandrasekaran et al. submitted). Bone morphogenetic protein (BMP) has been shown to control the graded expression of molecules that are implicated in the guidance of RGC axons and pattern the dorsal–ventral (DV) retinal axis (Plas et al. 2008). In BMP receptor (BMPr) mutant mice, dorsal RGC axons project ectopically to locations in the SC that normally receive input from more ventral retinal regions. In vivo, we observe a mixture of physiological phenotypes in SC neurons from BMPr mutant mice. These include ectopic, split, enlarged and patchy/distorted receptive fields (Chandrasekaran et al. submitted). Regardless of this large variation in RF size, however, response homeostasis is maintained in BMPr mutant mice (Fig. 1B). A plot of RF peak response versus RF area in the log domain is well fitted by a line of slope −1 for BMPr mutant mice neurons (Fig. 1B, open circles, r2= 0.7) and for control neurons (Fig. 1B, filled circles, r2= 0.67), suggesting that the total integrated response of the cell is kept constant despite large differences in how receptive fields are constructed in individual neurons.
Cellular mechanisms for maintaining homeostasis
Postsynaptic Ca2+ dynamics have been implicated in the read-out of neuronal homeostasis, as increases and decreases in Ca2+ are correlated with changes in firing properties (Turrigiano et al. 1994, 1995). Interestingly, the calcium–calmodulin stimulated enzyme CaMKII is implicated in this process, as over-expression of αCaMKII decreases mEPSC frequency while increasing mEPSC amplitude in cultured hippocampal neurons (Thiagarajan et al. 2002). The homeostatic action of CaMKII activity can also mediate competition in cortical neurons, as a decrease in synapse number has been attributed to uncorrelated inputs, while only inputs that are able to drive the postsynaptic neuron and activate CaMKII become functionally and structurally enhanced (Pratt et al. 2003). These results reveal a role for Ca2+ levels in regulating homeostasis, and implicate calcium–calmodulin stimulated enzymes in the same pathway. We have previously shown that in mice lacking the calcium–calmodulin stimulated enzyme adenylate cyclase 1 (AC1−/−), retinotopic map formation in the SC is impaired (Plas et al. 2004). We have also shown that the loss of AC1 signalling leads to disrupted receptive fields and the retention of a greater number of retinal inputs onto SC neurons (Shah et al. submitted). Unlike β2−/− mice and BMPr mutant mice, however, we find that the total integrated response to visual stimuli is increased in AC1−/− mice and response homeostasis is not preserved (Shah et al. submitted). Our analysis shows that the linear relationship between RF peak response and RF area, apparent in littermate controls (Fig. 1C, Control r2= 0.53), did not hold in AC1−/− neurons (Fig. 1C, AC1−/−r2= 0.21). We conclude that AC1 signalling is required for the activity dependent regulation of total synaptic input. These results identify AC1 as a potential molecular mechanism for cellular homeostasis in SC neurons and implicate AC1 in activity-dependent competition as well as in the read-out of overall activity during developmental changes in functional connectivity.
Synaptic mechanisms for maintaining homeostasis: learning rules and metaplasticity
Another mechanism for preserving the stability of neuronal responses is based on the concept of ‘metaplasticity’. In metaplasticity, the history of activity at any given synapse can regulate the capacity for further plasticity at that synapse (Bienenstock et al. 1982; Abraham & Bear, 1996). Synapses would have a variable modification threshold, and if their responses are previously strengthened, then their threshold for further LTP is increased, and vice versa. This process therefore could constrain synaptic weights within a narrow range. Over the last decade, this ‘sliding threshold’ model for synaptic plasticity has gained experimental support. In the hippocampus and visual cortex, for example, the sign and magnitude of plasticity correlates with initial synaptic strength, with weaker synapses showing LTP and stronger synapses showing LTD (Bi & Poo, 1998; Montgomery et al. 2001; Hardingham et al. 2007). Accordingly, neural activity during development can dynamically gate the threshold for plasticity by regulating synaptic maturation. For example, sensory deprivation experiments that result in a delayed maturation of cortical synapses also result in a greater potential for LTP at these immature synapses (Kirkwood et al. 1995, 1996; Franks & Isaacson, 2005). Our work at the retinocollicular synapse has revealed that young synaptic populations that are immature and weak on average also show LTP on average, while more mature populations that have strengthened over development do not (Shah & Crair, 2008). Furthermore, the lack of patterned retinal waves in β2−/− mice prevents the normal developmental strengthening of retinocollicular synapses. Interestingly, the capacity for LTP is preserved in these immature and weak synapses (Shah & Crair, 2008). These results reveal that dynamic, activity-dependent regulation of the capacity for synaptic plasticity may also play a part in keeping SC neuron output within an effective range.
Response homeostasis through regulation of synapse number and strength
Neurons can also restrict their output by regulating the number and strength of their synaptic inputs. Experiments at the neuromuscular junction (NMJ) have revealed numerous cell-autonomous mechanisms that serve to keep NMJ transmission constant in the face of altered activity (Davis & Goodman, 1998b). When the number of synapses is decreased genetically, there is a compensatory increase in strength of individual motoneuron synapses (Schuster et al. 1996; Davis & Goodman, 1998a). Conversely, when synaptic strength is genetically reduced postsynaptically, there is a compensatory increase in presynaptic transmitter release to preserve the level of transmission (Davis et al. 1998; Paradis et al. 2001). These results suggest that homeostatic mechanisms adjust NMJ transmission over development as synaptic competition and plasticity drive changes in the number and strength of inputs.
Developmental regulation of the number and strength of inputs has also been observed in the visual system of a number of species (Katz & Shatz, 1996). Over the first 3 postnatal weeks in the mouse LGN, retinal inputs are pruned and the AMPA/NMDA ratios and AMPA miniature amplitudes of the remaining retinal inputs are concurrently increased (Chen & Regehr, 2000). In the developing frog retinotectal system, excitatory visual receptive fields are initially large and single fibre retinal inputs are small. Over the course of development, RF areas decrease and the remaining retinal inputs are strengthened (Tao & Poo, 2005).
Our observations at the mouse retinocollicular synapse support a similar model of activity-dependent development. Using in vitro techniques, we have shown that response homeostasis in β2−/− animals is associated with modulation of the number and strength of retinal inputs (Chandrasekaran et al. 2007). In young adults aged P21–25, β2−/− SC neurons sample a similar number of equal strength retinal inputs relative to controls. Interestingly, homeostasis is also maintained earlier in development at P6–7 in β2−/− SC neurons, but via a different mechanism. At this age, β2−/− SC neurons sample a larger number of weaker strength retinal inputs than controls (Chandrasekaran et al. 2007). Combined, these studies suggest that homeostatic mechanisms act in concert with activity-dependent learning rules in a diverse range of neural systems to permit the fine-scale refinement of functional connectivity during development while preserving output response within an effective range.
Homeostatic regulation of intrinsic excitability
As described above, we find that the regulation of retinocollicular synapse number and strength is sufficient to explain the response homeostasis we observe in vivo. However, it is possible that changes in intrinsic excitability also contribute to the maintenance of response homeostasis. Many elegant studies have shown that neurons are capable of maintaining a constant firing rate despite changes in activity by regulating the rich array of voltage-gated conductances that they express (Zhang & Linden, 2003). This regulation can influence postsynaptic responsiveness (intrinsic excitability) by changing the threshold for spike generation and variability in interspike intervals. In cultured cortical neurons, for example, 48 h of activity blockade lowered the spike threshold and increased firing rate by selectively increasing voltage-gated sodium currents and decreasing persistent potassium currents. Interestingly, these conductance changes occurred on a similar time scale to the overall homeostatic changes in firing rate, suggesting a mechanistic link (Desai et al. 1999).
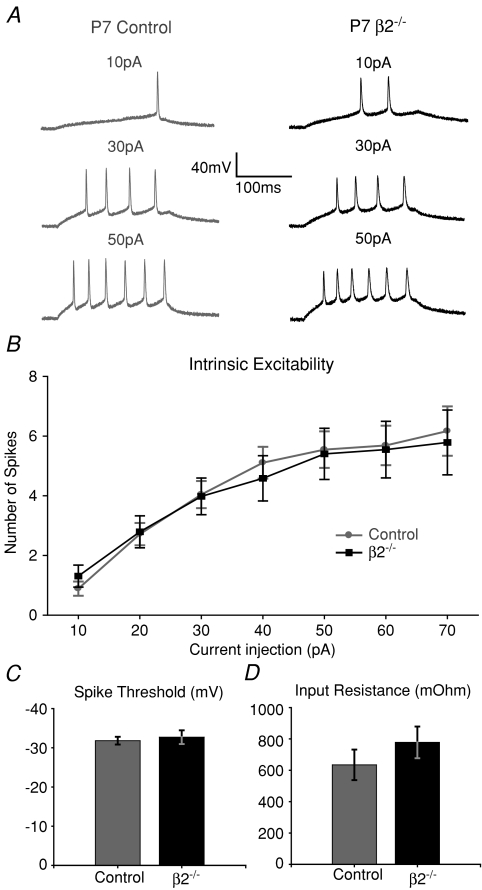
Studies in the retinotectal system of frogs have shown that intrinsic excitability is developmentally regulated (Aizenman et al. 2003; Pratt & Aizenman, 2007). A transient increase in tectal cell excitability occurs via the regulation of voltage-gated sodium currents, and as total synaptic drive increases, excitability decreases (Pratt & Aizenman, 2007). This allows tectal neurons to maintain a constant input–output function regardless of changes in the magnitude and pattern of retinal inputs. Furthermore, this increase in excitability occurs during a period where tectal cell morphology is highly dynamic and the refinement of retinotectal receptive fields is occurring (Wu & Cline, 2003; Tao & Poo, 2005). As changes in intrinsic excitability can regulate the ability of Hebbian learning rules to modify synaptic weights (Debanne et al. 2003; Zhang & Linden, 2003), these results suggest that homeostatic changes in intrinsic excitability play an important role in the activity-dependent development of retinotopic maps. We have shown that at P6–7, β2−/− SC neurons sample a greater number of weaker retinal inputs than control SC neurons, and we proposed that this regulation is sufficient to mediate response homeostasis in vivo (Chandrasekaran et al. 2007). It remains unknown, however, to what extent changes in intrinsic excitability also contribute to response homeostasis and map formation in this circuit. To address this, we used an in vitro preparation to perform current clamp recording in control and β2−/− SC neurons in the superficial layers of the SC at P6–8 (see Methods). We held the membrane potential at around −60 mV and delivered a series of 200 ms long square-wave depolarizing current injections and measured the number of action potentials fired at each current amplitude. Figure 2A shows example spiking responses from a P7 control and a P7 β2−/− SC neuron at three different current steps. A summary of the averaged input–output curves for each genotype in shown in Fig. 2B. We did not observe any changes in intrinsic excitability (Fig. 2B), spike threshold (Fig. 2C), or input resistance (Fig. 2D) between control and β2−/− SC neurons, suggesting that such regulation does not contribute to maintaining response homeostasis in β2−/− SC neurons. One reason why we may not observe such differences is that the overall activity levels may not be sufficiently different between control and β2−/− retinocollicular networks to trigger changes in excitability. Changes in the pattern of activity, however, can initiate regulation of the number and strength of inputs (Chandrasekaran et al. 2007). It is worth noting, however, that changes in intrinsic excitability may play a role in regulating homeostasis at earlier ages than those examined here. Furthermore, subtle differences in the number and distribution of voltage-activated ion channels in SC neurons, like those responsible for IA or Ih, may not be apparent in our input–output curves in vitro, but may still be involved in regulating response homeostasis in vivo. Moreover, as we are randomly sampling from a heterogeneous population of neurons in the SC, it is possible that differences in spike firing in different cell types are averaged out.
Figure 2. Intrinsic excitability of control and β2−/− SC neurons is similar at P6–8.
A, example current clamp recordings showing spiking responses at different current injection intensities in a P7 control (left) and P7 β2−/− (right) SC neuron. B, averaged input–output curves plotting the number of action potentials elicited in response to 200 ms long square current pulses at various amplitudes. Control curve (n = 13) is shown in grey, β2−/− curve (n = 10) in black. No differences in the excitability of SC neurons were observed between genotypes (P > 0.4 for every current amplitude, Student's t test). C, no difference in the spike threshold was observed between genotypes (P = 0.6). D, input resistance measurements were also similar across control and β2−/− cells (P = 0.3).
Conclusions
The mechanisms of homeostatic plasticity are ubiquitous in developing neural circuits, owing to their essential role in maintaining network stability in the face of changing activity patterns and positive-feedback cycles of synaptic strengthening and weakening. Many elegant studies across numerous species and brain regions have revealed multiple forms of neuronal homeostasis, including synaptic scaling, metaplasticity, changes in synapse number and strength, and regulation of intrinsic excitability. Work from our laboratory has revealed the phenomenon of response homeostasis, which maintains the output of individual superior collicular neurons in vivo despite changes in activity patterns or large differences in functional connectivity during the construction of visual receptive fields. Further in vitro studies have supported a metaplastic model of retinocollicular map development and the dynamic regulation of retinocollicular synapse number and strength, suggesting that homeostatic mechanisms and synaptic learning rules are concurrently active during the refinement of sensory maps. We also identify adenylate cyclase 1 as a potential molecular mechanism for the readout of total synaptic activity. Combined, our observations provide exciting clues for further investigation into the role of calcium signalling in mediating response homeostasis, as well as a framework for probing the functional differences between Hebbian synaptic competition and neuronal homeostasis during sensory map development.
Methods
Mice lacking the β2 subunit of nicotinic acetylcholine receptors (β2−/− mice) were generated in the Beaudet laboratory at Baylor College of Medicine and back-crossed at least six generations onto the C57BL/6 background. Genotypes were determined by genomic PCR using primer sequences and amplification parameters described in Xu et al. (1999). All experiments were performed blind to genotype; control mice were wild-type and heterozygous littermates of β2−/− mice. Animals were treated in accordance with IACUC and Yale University School of Medicine guidelines.
In vitro measurement of intrinsic excitability
Mice were first anaesthetized with isoflurane (2–4%, AErrane from Baxter Healthcare, Deerfield, IL, USA) and then decapitated. Parasagittal slices were prepared at P6–8 and patch clamp recordings were obtained from neurons in the superficial layers of the superior colliculus (SGS) as previously described (Shah & Crair, 2008). Whole cell recording electrodes contained (in mm): 100 potassium gluconate, 20 KCl, 5 NaCl, 10 Hepes, 0.5 EGTA, 4 MgATP, 0.3 GTP, 7 phosphocreatine, pH 7.2–7.5, 290–310 mosmol. After obtaining a stable voltage-clamp recording to monitor input and series resistance, the recording was switched to current clamp. Resting membrane potential was adjusted to between −60 and −65 mV. A 200 ms long, square-wave current injection was delivered via the amplifier in order to depolarize the membrane. Current injections were given at 10 pA amplitude intervals from 10 to 70 pA, and 8–10 sweeps were collected for each amplitude. To analyse the number of spikes, we took the first derivative of each sweep and used a threshold of 30 mV ms−1 to define an action potential. The number of spikes elicited for each current injection amplitude was averaged for each cell, and the values were then averaged across experiments (Fig. 2). For the spike threshold analysis, we analysed the first spike elicited at various current injection amplitudes for each cell. Spike threshold was defined as the voltage at which dV/dt crossed 30 mV ms−1.
Acknowledgments
We thank Yueyi Zhang for technical assistance, Onkar Dhande for helpful discussions and members of the Crair lab for helpful comments on the manuscript. This work was supported, in part, by NIH grants R01 EY015788 and P30 EY000758.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci. 2005;25:6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007;27:1746–1755. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- Debanne D, Daoudal G, Sourdet V, Russier M. Brain plasticity and ion channels. J Physiol Paris. 2003;97:403–414. doi: 10.1016/j.jphysparis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005;47:101–114. doi: 10.1016/j.neuron.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the b2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40:1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Hardingham NR, Hardingham GE, Fox KD, Jack JJ. Presynaptic efficacy directs normalization of synaptic strength in layer 2/3 rat neocortex after paired activity. J Neurophysiol. 2007;97:2965–2975. doi: 10.1152/jn.01352.2006. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Pavlidis P, Madison DV. Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron. 2001;29:691–701. doi: 10.1016/s0896-6273(01)00244-6. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Plas DT, Dhande OS, Lopez JE, Murali D, Thaller C, Henkemeyer M, Furuta Y, Overbeek P, Crair MC. Bone morphogenetic proteins, eye patterning, and retinocollicular map formation in the mouse. J Neurosci. 2008;28:7057–7067. doi: 10.1523/JNEUROSCI.3598-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DT, Visel A, Gonzalez E, She WC, Crair MC. Adenylate cyclase 1 dependent refinement of retinotopic maps in the mouse. Vision Res. 2004;44:3357–3364. doi: 10.1016/j.visres.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Watt AJ, Griffith LC, Nelson SB, Turrigiano GG. Activity-dependent remodeling of presynaptic inputs by postsynaptic expression of activated CaMKII. Neuron. 2003;39:269–281. doi: 10.1016/s0896-6273(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Shah RD, Crair MC. Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves. J Neurosci. 2008;28:292–303. doi: 10.1523/JNEUROSCI.4276-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. α- and βCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, LeMasson G, Marder E. Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci. 1995;15:3640–3652. doi: 10.1523/JNEUROSCI.15-05-03640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Time-lapse in vivo imaging of the morphological development of Xenopus optic tectal interneurons. J Comp Neurol. 2003;459:392–406. doi: 10.1002/cne.10618. [DOI] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]