Abstract
The serotonin system in prefrontal cortex (PFC) is critically involved in the regulation of cognition and emotion. To understand the cellular mechanisms underlying its physiological actions, we investigated the role of serotonin in regulating synaptic plasticity in PFC circuits. We found that tetanic stimuli coupled to bath application of serotonin induced long-term depression (LTD) at excitatory synapses of PFC pyramidal neurons. This effect was mediated by 5-HT2A/C receptors and was independent of NMDA receptor activation. A group I metabotropic glutamate receptor (mGluR) antagonist blocked the LTD induction by serotonin + tetani, and co-application of a group I mGluR agonist and serotonin, but not application of either drug alone, induced LTD without tetani. The effect of serotonin on LTD was blocked by selective inhibitors of p38 mitogen-activated protein kinase (MAPK), but not p42/44 MAPK. Biochemical evidence also indicated that serotonin and a group I mGluR agonist synergistically activated p38 MAPK in PFC slices. The serotonin-facilitated LTD induction was prevented by blocking the activation of the small GTPase Rab5, as well as by blocking the clathrin-dependent internalization of AMPA receptors with postsynaptic injection of a dynamin inhibitory peptide, while it was unaffected by manipulating the cytoskeleton. Interestingly, in animals exposed to acute stress, the LTD induction by serotonin + tetani was significantly impaired. Taken together, these results suggest that serotonin, by cooperating with mGluRs, regulates synaptic plasticity through a mechanism dependent on p38 MAPK/Rab5-mediated enhancement of AMPA receptor internalization in a clathrin/dynamin-dependent manner. It provides a potential mechanism underlying the role of serotonin in controlling emotional and cognitive processes that are mediated by synaptic plasticity in PFC neurons.
Prefrontal cortex (PFC) is a brain region critical for many high-level, ‘executive’ processes, such as working memory, attention, inhibition of distraction, novelty seeking, emotional control, decision making and encoding of context (Stuss & Knight, 2002). One of the most important neuromodulators that powerfully influence PFC functions is serotonin (Davidson et al. 2000; Williams et al. 2002; Yan, 2002). Aberrant serotonergic neurotransmission has long been implicated in the pathogenesis of neuropsychiatric disorders that are associated with PFC dysfunction, including schizophrenia, depression and anxiety (Breier, 1995; Dubovsky & Thomas, 1995; Abi-Dargham et al. 1997; Buhot, 1997; Stockmeier, 1997; Gross & Hen, 2004). Because of the complexity of the 5-HT receptor subtypes (Martin et al. 1998) distributed within the neuronal circuits of PFC (Goldman-Rakic et al. 1990; Feng et al. 2001), relatively little is known about the functional role of serotonin in PFC. The two most abundant 5-HT receptor subtypes in PFC, 5-HT1A and 5-HT2A, are enriched in postsynaptic dendritic shafts and dendritic spines of pyramidal neurons (Kia et al. 1996; Jakab & Goldman-Rakic, 1998) where glutamate receptors are concentrated, raising the possibility that serotonin may exert some of its functions by modulating glutamatergic synapses (Aghajanian & Marek, 1997; Cai et al. 2002; Yuen et al. 2005).
In both invertebrate and vertebrate nervous systems, glutamatergic synaptic transmission can undergo long-term changes in efficacy, a phenomenon called synaptic plasticity (Collingridge & Singer, 1990; Siegelbaum & Kandel, 1991; Malenka & Nicoll, 1999). The two most widely known examples of activity-dependent synaptic plasticity of excitatory transmission, long-term potentiation (LTP) and long-term depression (LTD), are leading synaptic models for experience-induced modification of brain function, such as learning and memory (Malenka & Bear, 2004). It has been found that the gating and the polarity of synaptic plasticity in cortex can be controlled by neuromodulators (Otani et al. 1998; Matsuda et al. 2006; Seol et al. 2007). Serotonin can affect the induction of LTP and LTD in a complicated manner, depending on the different 5-HT receptor subtypes, brain regions and developmental stages (Kojic et al. 1997; Edagawa et al. 2000, 2001; Kemp & Manahan-Vaughan, 2004). Administration of selective serotonin reuptake inhibitors also gives variable effects on synaptic plasticity, with the LTP induction in CA1 hippocampus being blocked (Shakesby et al. 2002), and LTP in the hippocampo-medial PFC pathway being significantly augmented (Ohashi et al. 2002). Moreover, it has been found that serotonin promotes the probability of LTP in 5-HT2C receptor-rich zones and facilitates LTD induction in 5-HT2C receptor-poor zones of visual cortex (Kojic et al. 2000), suggesting that serotonin may control not only whether plasticity occurs, but also where a given input is strengthened or weakened (Kirkwood, 2000).
In this study, we examined the impact of serotonin on synaptic plasticity of glutamatergic transmission in PFC pyramidal neurons, which could provide a potential cellular mechanism underlying the serotonergic regulation of cognitive processes associated with normal mental function and neuropsychiatric disorders.
Methods
Electrophysiological recordings in slices
Pyramidal neurons located in deep layers (V–VI) of the PFC of Sprague–Dawley rats (3–5 weeks postnatal) were recorded. All experiments were carried out with the approval of the State University of New York at Buffalo Animal Care Committee. Slice preparation procedures were similar to what was described before (Zhong et al. 2003; Tan et al. 2004; Yuen et al. 2005). In brief, animals were anaesthetized by inhaling 2-bromo-2-chloro-1,1,1-trifluoroethane (1 ml (100 g)−1, Sigma) and decapitated. Brains were quickly removed and sliced (300–400 μm) with a Leica VP1000S Vibrotome while bathed in a Hepes-buffered salt solution. Slices were then incubated for 1–5 h at room temperature (22–24°C) in a NaHCO3-buffered saline bubbled with 95% O2–5% CO2.
To measure excitatory postsynaptic currents in PFC slices, the whole-cell voltage-clamp recording technique was used (Zhong et al. 2003; Zhong & Yan, 2004). Electrodes (5–9 MΩ) were filled with the following internal solution (in mm): 130 caesium methane-sulphonate, 10 CsCl, 4 NaCl, 10 Hepes, 1 MgCl2, 5 EGTA, 2.2 QX-314, 12 phosphocreatine, 5 MgATP, 0.2 Na3GTP, 0.1 leupeptin; pH 7.2–7.3; 265–270 mosmol l−1. The slice (300 μm) was placed in a perfusion chamber attached to the fixed stage of an upright microscope (Olympus) and submerged in continuously flowing oxygenated artificial cerebrospinal fluid (ACSF, 130 mm NaCl, 26 mm NaHCO3, 2 mm CaCl2, 5 mm MgCl2, 3 mm KCl, 10 mm glucose, 1.25 mm NaH2PO4). Cells were visualized with a ×40 water-immersion lens and illuminated with near infrared (IR) light and the image was detected with an IR-sensitive CCD camera. A Multiclamp 700A amplifier was used for these recordings. Tight seals (2–10 GΩ) from visualized pyramidal neurons were obtained by applying negative pressure. Recordings were done at room temperature. The membrane was disrupted with additional suction and the whole cell configuration was obtained. The access resistances ranged from 13 to 18 MΩ and were compensated 50–70%. For the recording of AMPAR-mediated eEPSCs, cells (voltage-clamped at −70 mV) were bathed in ACSF containing bicuculline (2 μm) to block GABAA receptors. A bipolar stimulating electrode (FHC) was positioned ∼100 μm from the neuron being recorded. Evoked currents were generated with a 50 μs test pulse from a stimulation isolation unit controlled by a S48 pulse generator (Astro-Medical). LTD-inducing tetanic stimuli consisted of four trains of 50 Hz stimuli (100 pulses per train), delivered at 0.1 Hz. The 0.033 Hz test stimuli (1 pulse (30 s)−1) were resumed 30 s after tetanic stimulation. The averaged responses from the 10 min period just before tetani–drug application and the 50–60 min period after tetani–drug application were compared to express changes of the eEPSC amplitude.
Serotonin receptor ligands 5-HT, α-Me-5-HT, (–)-2, 5-dimethoxy-4-iodoamphetamine (DOI), ketanserin, cyanopindolol, and actin/microtubule agents phalloidin and taxol (Sigma), as well as second messenger reagents SB203580, PD98059 and U0126 (Calbiochem) were made up as concentrated stock solutions in water or DMSO and stored at −20°C. Stocks were thawed and diluted immediately prior to use. The amino acid sequence for the dynamin inhibitory peptide (Tocris) is: QVPSRPNRAP. The polyclonal anti-Rab5 antibody (Santa Cruz) was raised against the full-length human Rab5A. Wild-type and mutant (S34N and Q79L) Rab5 proteins were generated as previous described (Chen et al. 2007). Data analyses were performed with Clampfit (Axon Instruments), Origin 6 (OriginLab) and Kaleidagraph (Albeck Software). ANOVA tests were performed to compare groups subjected to different treatment.
Western blot analysis
PFC slices were prepared as previously described (Gu et al. 2003; Gu & Yan, 2004). After drug treatment, equal amounts of protein from slice homogenates were separated on 7.5% acrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% non-fat dried milk for 1 h at room temperature. Then the blots were incubated with the phospho-p38 MAPK (Thr180/Tyr182) antibody (1 : 1000, Cell Signaling) for 1 h at room temperature. After being rinsed, the blots were incubated with horseradish peroxidase-conjugated anti-rabbit antibodies (Amersham, 1 : 2000) for 1 h at room temperature. Following three washes, the blots were exposed to the enhanced chemiluminescence substrate. Then the blots were stripped for 1 h at 50°C followed by saturation in 5% non-fat dried milk and incubated with the p38 MAPK antibody (1 : 1000, Cell Signaling). Quantification was obtained from densitometric measurements of immunoreactive bands on films.
Stress protocol
Two stress protocols (elevated platform test and forced swim test) as previously described (Xu et al. 1997; Roche et al. 2003; Tan et al. 2004) were used in our studies. In the elevated platform tests, rats were placed on an elevated platform (20 cm × 20 cm) for 20 min in a brightly lit room. The animal showed behavioural ‘freezing’ (i.e. immobility) for up to 10 min. In the swim tests, rats were placed in a cylindrical glass tank (24.5 cm high × 18.5 cm diameter) filled with water to a depth of 20 cm. Rats were forced to swim in warm water (24–26°C) for 20 min. About 30 min after the stress procedures, rats were anaesthetized and killed. Experimental groups were matched so that a stressed rat and a control rat were perfused on the same day and tissue was processed in parallel.
Results
Serotonin, by activating 5-HT2A/2C receptors, facilitates LTD induction in prefrontal cortex
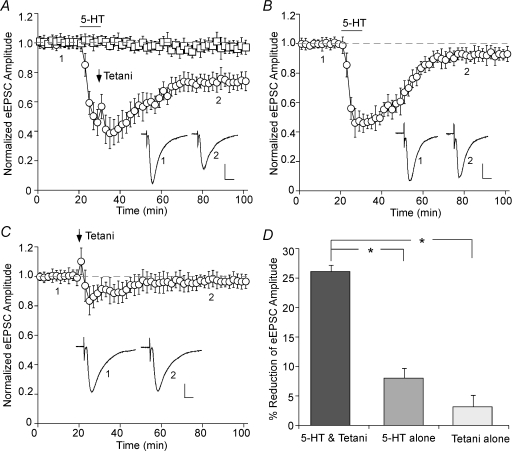
To test the role of serotonin in the regulation of synaptic plasticity, we examined the effect of 5-HT on glutamatergic responses in PFC pyramidal neurons. As shown in Fig. 1A, stable AMPA receptor-mediated EPSCs were evoked by short test pulses (delivered in 30 s intervals) throughout the recording (n = 10). However, tetanic stimuli (100 pulses at 50 Hz, repeated four times in 10 s intervals) delivered at the end of a 10 min bath application of 5-HT (40 μm) induced long-term depression (LTD) of the EPSC amplitude. 5-HT alone only transiently depressed the EPSC (Fig. 1B). Tetanic stimulation alone also failed to induce long-term changes of the synaptic response (Fig. 1C). As summarized in Fig. 1D, a combination of serotonin application and the 50 Hz tetani induced 26.1 ± 1.1% (n = 18) reduction of the EPSC amplitude (measured at 50–60 min after washout), while serotonin alone or tetani alone had little long-term effect (5-HT: 8.3 ± 1.6%, n = 12; tetani: 3.2 ± 1.9%, n = 6).
Figure 1. Serotonin facilitates LTD induction in PFC pyramidal neurons.
A, plot of normalized eEPSC amplitude in PFC neurons with or without 5-HT (40 μm, 10 min) application coupled to 50 Hz tetanic stimuli (added 9 min after the start of 5-HT application). Each point is the average (mean ±s.e.m.) of four responses. Note the induction of LTD by 5-HT + tetani. B, plot of normalized eEPSC amplitude in PFC neurons subjected to 5-HT (40 μm, 10 min) application. Note that the synaptic responses were acutely suppressed by bath application of 5-HT, but they fully recovered within 40 min after 5-HT washout. C, plot of normalized eEPSC amplitude in PFC neurons subjected to 50 Hz tetani application. Note that the synaptic responses were largely unchanged by tetanic stimuli. Inset (A–C): averaged synaptic responses taken from the indicated time points in neurons subjected to 5-HT + tetani application (A), 5-HT alone (B), or tetanic stimuli alone (C). Scale bars: 50 pA, 10 ms. D, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by different treatments (measured at 50–60 min after washout). *P < 0.005, ANOVA.
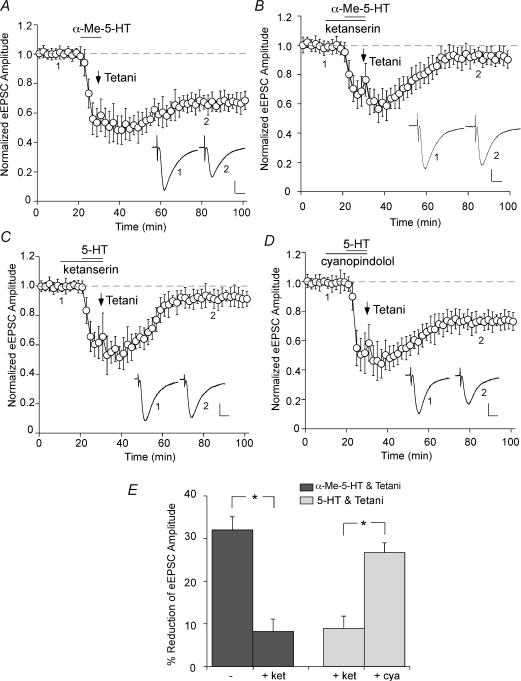
Our previous study has shown that 5-HT1A and 5-HT2A receptors are the most predominant serotonin receptor subtypes expressed in PFC pyramidal neurons (Feng et al. 2001), thus we examined the role of these receptors in serotonergic facilitation of LTD. Co-application of the specific 5-HT2A/2C agonist α-Me-5-HT (20 μm) and 50 Hz tetani caused a sustained reduction in the EPSC amplitude (Fig. 2A; 32.0 ± 3.1%, n = 5; Fig. 2E), mimicking the LTD induction by 5-HT + tetani. Another 5-HT2A/2C agonist (–)DOI (50 μm) induced similar LTD when coupled with tetani (29.6 ± 3.2%, n = 3). In the presence of ketanserin (20 μm), a selective 5-HT2A/2C antagonist, LTD failed to be induced by α-Me-5-HT + tetani (Fig. 2B; 8.2 ± 2.9%, n = 3; Fig. 2E) or 5-HT + tetani (Fig. 2C; 8.9 ± 3.0%, n = 5; Fig. 2E). However, the 5-HT1-class antagonist cyanopindolol (10 μm) was ineffective in blocking LTD induction by 5-HT + tetani (Fig. 2D; 26.7 ± 2.3%, n = 5; Fig. 2E). The pharmacological data thus suggest that serotonin released on PFC pyramidal neurons could modulate synaptic plasticity via the activation of 5-HT2A/2C receptors.
Figure 2. 5-HT2A/2C receptors mediate the effect of serotonin on LTD induction.
A, plot of normalized eEPSC amplitude in PFC neurons subjected to co-application of the 5-HT2A/2C agonist α-Me-5-HT (20 μm, 10 min) and 50 Hz tetanic stimuli. Note the induction of LTD by α-Me-5-HT + tetani. B and C, plot of normalized eEPSC amplitude in PFC neurons pretreated with the 5-HT2A/2C antagonist ketanserin (20 μm) and subjected to α-Me-5-HT + tetani (B) or 5-HT + tetani (C) application. D, plot of normalized eEPSC amplitude in PFC neurons pretreated with the 5-HT1 antagonist cyanopindolol (10 μm) and subjected to 5-HT + tetani application. Note that ketanserin, but not cyanopindolol, blocked the LTD induction. Inset (A–D): averaged synaptic responses taken from the indicated time points. Scale bars: 50 pA, 10 ms. E, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by different treatments (measured at 50–60 min after washout). *P < 0.005, ANOVA.
The serotonergic facilitation of LTD induction involves group I mGluR activation
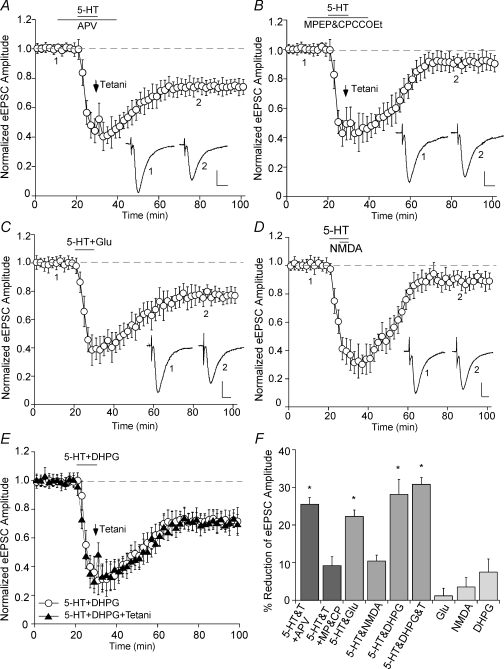
We next tested whether the LTD induction by serotonin and tetanic stimulation requires the activation of specific glutamate receptors. As shown in Fig. 3A, in the presence of the NMDAR antagonist APV (50 μm), the LTD induction by 5-HT and tetani was intact (25.5 ± 1.8%, n = 9; Fig. 3F), suggesting the lack of involvement of NMDA receptors in this synaptic plasticity. In contrast, when group I mGluRs were blocked by their antagonists MPEP (10 μm) and CPCCOEt (50 μm), co-application of 5-HT and tetani failed to induce LTD (Fig. 3B; 9.2 ± 2.4%, n = 6; Fig. 3F). A combined application of 5-HT and glutamate (50 μm) induced LTD without tetani (Fig. 3C; 22.3 ± 1.7%, n = 5; Fig. 3F), while co-application of 5-HT and NMDA (20 μm) was ineffective in inducing significant LTD (Fig. 3D; 10.4 ± 1.5%, n = 6; Fig. 3F). Moreover, a combined 10 min application of 5-HT and the group I mGluR agonist DHPG (100 μm) induced LTD without tetani (Fig. 3E; 28.1 ± 4.0%, n = 5; Fig. 3F). Compared to the LTD induced by 5-HT + DHPG or by 5-HT + tetani, when 5-HT is coupled with both DHPG and tetani, no additive LTD was induced (Fig. 3E; 30.8 ± 1.8%, n = 3, Fig. 3F), suggesting that DHPG occluded tetani in 5-HT-facilitated LTD induction. Glutamate, NMDA or DHPG itself was incapable of inducing sustained changes of the EPSC amplitude (Fig. 3F). These data suggest that glutamate released from tetanic stimulation activates the group I mGluR, which is necessary and sufficient for the LTD induction in the presence of serotonin.
Figure 3. The LTD induction by serotonin and tetani is independent of NMDA receptors, but depends on group I mGluR activation.
A and B, plot of normalized eEPSC amplitude in PFC neurons pretreated with the NMDAR antagonist APV (50 μm, A) or the group I mGluR antagonist MPEP (10 μm) plus CPCCOEt (50 μm) (B) and subjected to 5-HT + tetani application. Note that MPEP plus CPCCOEt, but not APV, blocked the LTD induction. C and D, plot of normalized eEPSC amplitude in PFC neurons subjected to co-application of 5-HT (40 μm) and glutamate (50 μm, C) or NMDA (20 μm, D). E, plot of normalized eEPSC amplitude in PFC neurons subjected to co-application of 5-HT (40 μm) and the group I mGluR agonist DHPG (100 μm) with or without coupling to tetani. Note the induction of LTD by 5-HT + glutamate or 5-HT + DHPG, but not 5-HT + NMDA. Inset (A–E): averaged synaptic responses taken from the indicated time points. Scale bars: 50 pA, 10 ms. F, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by different treatments (measured at 50–60 min after washout). *P < 0.005, ANOVA.
p38 MAP kinase mediates the serotonin effect on LTD induction
In the following set of experiments, we sought to find out the mechanisms underlying serotonin-facilitated LTD induction in PFC. To examine the pre- versus postsynaptic origin of this synaptic plasticity, we measured the ratio of paired-pulse facilitation (PPR), an index of presynaptic processes, before and after LTD induction. No significant change was observed in PPR (before LTD: 2.05 ± 0.13; after LTD: 2.11 ± 0.14, n = 4, P > 0.5, ANOVA), suggesting that the LTD induction is probably through a postsynaptic mechanism.
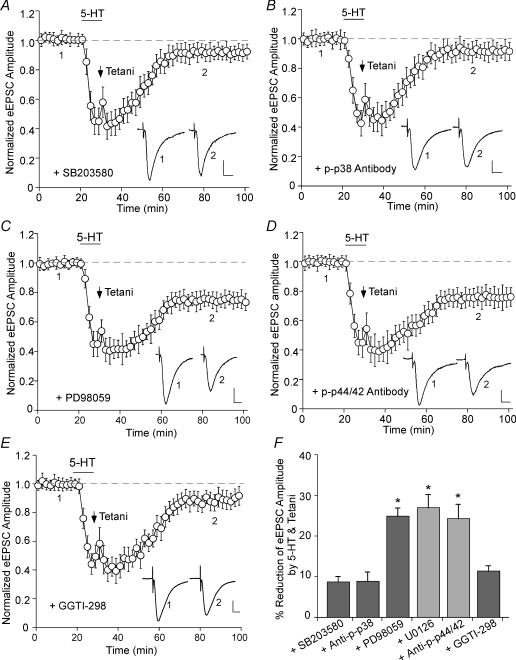
Previous studies have found that MAP kinases are involved in various forms of synaptic plasticity (Bolshakov et al. 2000; Zhu et al. 2002; Rush et al. 2002; Guan et al. 2003). Thus, we applied selective MAP kinase inhibitors and examined the LTD induction by 5-HT and tetani. As shown in Fig. 4A, postsynaptic injection of the specific p38 MAPK inhibitor SB203580 (Lee et al. 1999; 20 μm) prevented the LTD induction (8.7 ± 1.1%, n = 15; Fig. 4F). Moreover, dialysis with an affinity-purified antibody raised against activated (Thr180/Tyr182-phosphorylated) p38 MAPK (1 : 100) also blocked the LTD induction (Fig. 4B; 8.9 ± 2.2%, n = 5; Fig. 4F). On the other hand, postsynaptic injection of PD98059 (40 μm), a selective inhibitor of MEK, the p42/44 MAPK-activating enzyme (Dudley et al. 1995), did not affect the LTD induction (Fig. 4C; 24.9 ± 1.9%, n = 10; Fig. 4F). Another structurally different selective p42/44 MAPK kinase inhibitor U0126 (20 μm) gave similar results, i.e. failed to block the LTD induction (27.0 ± 3.1%, n = 6; Fig. 4F). Moreover, dialysis with an antibody against active (Thr202/Tyr204-phosphorylated) p42/44 MAPK (1 : 100) was also ineffective in blocking the LTD induction (Fig. 4D; 24.3 ± 3.5%, n = 3; Fig. 4F). These results suggest that serotonergic facilitation of LTD induction in PFC requires activation of p38, but not p42/44, MAP kinase.
Figure 4. The serotonergic facilitation of LTD induction requires activation of p38 MAP kinase, but not p42/44 MAP kinases.
A and B, plot of normalized eEPSC amplitude in PFC neurons dialysed with the selective p38 MAPK inhibitor SB203580 (20 μm, A) or an antibody against active (Thr180/Tyr182-phosphorylated) p38 MAPK (1 : 100, B) and subjected to 5-HT + tetani application. Note the block of LTD induction with these p38 MAPK inhibitors. C and D, plot of normalized eEPSC amplitude in PFC neurons dialysed with the selective p42/44 MAPK inhibitor PD98059 (40 μm, C) or an antibody against active (Thr202/Tyr204-phosphorylated) p42/44 MAPK (1 : 100, D) and subjected to 5-HT + tetani application. Note that these p42/44 MAPK inhibitors failed to block LTD induction. E, plot of normalized eEPSC amplitude in PFC neurons dialysed with the Rap1 inhibitor GGTI-298 (20 μm) and subjected to 5-HT + tetani application. Inset (A–E): averaged synaptic responses taken from the indicated time points. Scale bars: 50 pA, 10 ms. F, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by 5-HT + tetani (measured at 50–60 min after washout) in the presence of different agents. *P < 0.005, ANOVA.
To further test the requirement of p38 MAP kinase, we blocked one of its upstream activators, the small GTPase Rap1 (Huang et al. 2004), and examined the LTD induction by 5-HT and tetani. PFC slices were pretreated with the Rap1 inhibitor GGTI-298 (20 μm) for more than 1 h, and neurons were recorded with GGTI-298 in the pipette. As shown in Fig. 4E, GGTI-298 largely blocked the LTD induction (11.4 ± 1.2%, n = 3; Fig. 4F).
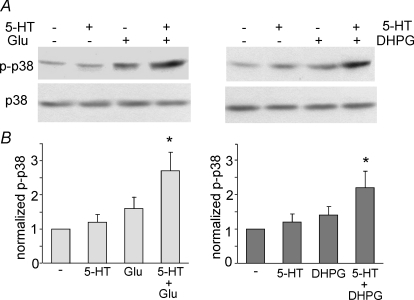
To provide more evidence on the involvement of p38 MAPK in the serotonin-faciliated LTD induction, we measured p38 MAPK activation in PFC slices by protocols that can or cannot induce LTD. Activation of p38 MAPK occurs through a dual phosphorylation at threonine 180 and tyrosine 182 (Raingeaud et al. 1995), thus a phospho-p38 MAPK (Thr180/Tyr182) antibody was used to detect activated p38 MAPK. As shown in Fig. 5A and B, treatment of PFC slices with 5-HT (20 μm, 10 min) alone failed to induce p38 MAPK activation (1.2 ± 0.2 fold, n = 4). Glutamate (50 μm, 10 min) alone had a small effect on p38 activation (1.6 ± 0.3 fold, n = 4). However, a combined application of 5-HT and glutamate induced a strong activation of p38 MAPK (2.7 ± 0.6 fold, n = 4, P < 0.005, ANOVA). Similarly, co-application of 5-HT (20 μm, 10 min) with the group I mGluR agonist DHPG (100 μm, 10 min) strongly activated p38 MAPK (2.2 ± 0.5 fold, n = 4, P < 0.005, ANOVA), despite the minimal effect of DHPG itself (1.4 ± 0.3 fold, n = 4). The level of total p38 MAPK was not changed by any of these treatments. These data indicate that the LTD induction by 5-HT and tetani (or group I mGluR) involves a synergistic activation of p38 MAPK.
Figure 5. Co-activation of serotonin receptors and mGluRs synergistically activates p38 MAP kinase in PFC slices.
A, Western blot analysis of activated (Thr180/Tyr182-phosphorylated) p38 MAPK (top) and total p38 MAPK (bottom) in lysates of PFC neurons treated without (–) or with 10 min of 5-HT (40 μm), glutamate/glycine (Glu, 50 μm/5 μm) or 5-HT + Glu (left). Alternatively, PFC neurons were treated without (–) or with 10 min of 5-HT (40 μm), DHPG (100 μm), or 5-HT + DHPG (right). Note the strong activation of p38 with 5-HT co-applied with Glu or DHPG. B, quantification of p38 activation with different treatments. Each bar represents mean ±s.e.m. of 4–5 independent experiments. *P < 0.005, ANOVA.
The serotonergic facilitation of LTD induction requires the Rab5-mediated internalization of AMPA receptors in a clathrin/dynamin-dependent manner
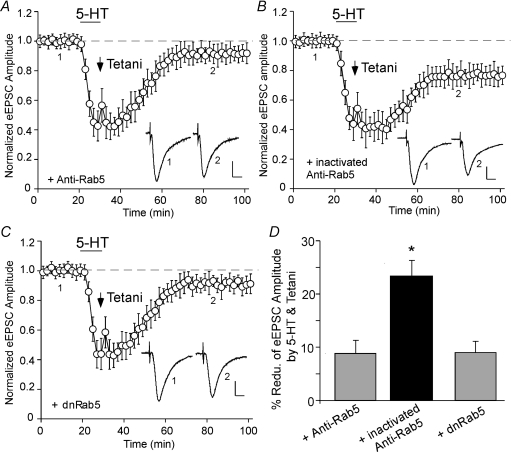
Next, we searched for the signalling pathway downstream of p38 MAPK to induce synaptic depression. Recent evidence has shown that p38 MAPK is capable of regulating endocytic trafficking via activation of the small GTPase Rab5 (Cavalli et al. 2001; Huang et al. 2004), a key mediator of protein transport from plasma membrane to early endosomes during clathrin-dependent endocytosis (Bucci et al. 1992; de Hoop et al. 1994). To test the role of Rab5 in serotonin-facilitated LTD induction, we dialysed postsynaptic neurons with an antibody against Rab5 that can cause inactivation of endogenous, membrane-associated Rab5 (Gorvel et al. 1991). As shown in Fig. 6A, the LTD induction by 5-HT + tetani was blocked in neurons loaded with the Rab5 antibody (8.8 ± 2.5%, n = 6; Fig. 6D). In contrast, the heat-inactivated Rab5 antibody, which does not bind to Rab5 and has lost the competence to inhibit Rab5, failed to affect the LTD induction (Fig. 6B; 23.4 ± 4.1%, n = 3; Fig. 6D). To further examine the role of Rab5, we injected neurons with the purified dominant-negative (dn) mutant form of Rab5 protein (Rab5S34N), which inhibits clathrin-mediated endocytosis (Stenmark et al. 1994). As shown in Fig. 6C, the serotonin-facilitated induction of LTD in PFC was significantly diminished in dnRab5-loaded cells (8.9 ± 2.1%, n = 6; Fig. 6D). Moreover, we injected neurons with the purified constitutively active (ca) variant of Rab5 protein (Rab5Q79L), which accelerates endocytosis (Stenmark et al. 1994). We found that caRab5 caused a gradual depression of eEPSC amplitude (21.0 ± 2.1%, n = 4), and largely occluded the effect of serotonin + tetani application (9.3 ± 2.5%, n = 5; data not shown). These results suggest that Rab5 activity is required for the serotonin-facilitated PFC synaptic plasticity.
Figure 6. Activation of the small GTPase Rab5, a key mediator of receptor endocytosis and endosomal dynamics, is required for the LTD induction by serotonin and tetani.
A–C, plot of normalized eEPSC amplitude in PFC neurons injected with an anti-Rab5 antibody (2 μg ml−1, A), a heat-inactivated anti-Rab5 antibody (2 μg ml−1, B) or the purified dominant negative Rab5 (dnRab5) protein (4 μg ml−1, C) and subjected to 5-HT + tetani application. Note the block of LTD induction with anti-Rab5 or dnRab5. Inset (A–C): averaged synaptic responses taken from the indicated time points. Scale bars: 50 pA, 10 ms. D, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by 5-HT + tetani (measured at 50–60 min after washout) in the presence of different agents. *P < 0.005, ANOVA.
Since Rab5 provides a potential link between p38 MAPK and AMPAR trafficking, we further tested the involvement of clathrin-dependent endocytosis of AMPA receptors in the serotonin-facilitated LTD induction. To do so, we dialysed neurons with a dynamin inhibitory peptide that interferes with the binding of amphiphysin with dynamin and therefore prevents clathrin-dependent endocytosis (Gout et al. 1993; Grabs et al. 1997; Marks & McMahon, 1998). Previous studies have shown that this peptide can block AMPAR endocytosis and hippocampal LTD (Luscher et al. 1999; Morishita et al. 2005). As shown in Fig. 7A, the LTD induction by 5-HT and tetani was blocked in neurons loaded with the dynamin inhibitory peptide (100 μm, 9.7 ± 2.7%, n = 6; Fig. 7E), but not a scrambled control peptide (100 μm, Fig. 7B and 25.6 ± 4.1%, n = 3; Fig. 7E), suggesting that the clathrin-mediated AMPAR endocytosis is required for the LTD induction by 5-HT and tetani.
Figure 7. The serotonergic facilitation of LTD induction is mediated by AMPA receptor internalization in a clathrin/dynamin-dependent and cytoskeleton-independent manner.
A and B, plot of normalized eEPSC amplitude in PFC neurons dialysed with a dynamin inhibitory peptide (100 μm, A) or a scrambled control peptide (100 μm, B) and subjected to 5-HT + tetani application. Note the block of LTD induction with the dynamin inhibitory peptide. C and D, plot of normalized eEPSC amplitude in PFC neurons dialysed with the actin stabilizer phalloidin (4 μm, C) or the microtubule stabilizer taxol (10 μm, D) and subjected to 5-HT + tetani application. Note that these cytoskeleton agents failed to block the LTD induction. Inset (A–D): averaged synaptic responses taken from the indicated time points. Scale bars: 50 pA, 10 ms. E, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by 5-HT + tetani (measured at 50–60 min after washout) in the presence of different agents. *P < 0.005, ANOVA.
Because Rab5 activity has also been linked to actin remodelling (Lanzetti et al. 2004), and cytoskeleton integrity has been implicated in AMPA receptor trafficking (Zhou et al. 2001; Ives et al. 2004), we next tested the role of actin and microtubule networks in the serotonin-facilitated LTD induction. As shown in Fig. 7C, dialysis with phalloidin (4 μm), an actin-stabilizing compound, failed to affect the LTD induction by 5-HT and tetani (22.4 ± 3.0%, n = 6; Fig. 7E). Similarly, the tetani-induced LTD in the presence of 5-HT was also intact in neurons loaded with taxol (10 μm), a microtubule-stabilizing agent (Fig. 7D; 21.5 ± 2.6%, n = 6; Fig. 7E). These data suggest that the LTD induction by 5-HT and tetani is not dependent on the stability of actin filaments or microtubule assembly.
The serotonergic facilitation of LTD induction is impaired in stressed animals
To understand the potential implication of the serotonin-facilitated PFC synaptic plasticity in cognitive and emotional processes, we examined LTD induction by 5-HT and tetani in animals exposed to stress, since many mental illnesses are exacerbated by stress (Mazure, 1995). The stress procedures we used entailed placing the rat on the elevated platform for 20 min (Xu et al. 1997), followed by forcing the rat to swim in deep water for 20 min (Roche et al. 2003; Tan et al. 2004). As shown in Fig. 8A and B, the LTD induction by 5-HT and tetani was substantially diminished in PFC neurons from stressed rats (11.5 ± 2.4%, n = 6), compared to PFC neurons from non-stressed control rats (25.7 ± 2.5%, n = 6). It suggests that the serotonin-regulated long-term plasticity is critically involved in mental processes subserved by PFC circuits.
Figure 8. The serotonergic facilitation of LTD induction is impaired in animals exposed to stress.
A, plot of normalized eEPSC amplitude subjected to 5-HT + tetani application in PFC neurons from stressed and control (non-stressed) rats. Note the diminished LTD induction in animals exposed to stress protocols (elevated platform and forced swim). Inset: averaged synaptic responses taken from the indicated time points. Scale bars: 50 pA, 10 ms. B, cumulative data (mean ±s.e.m.) showing the percentage reduction of eEPSC by 5-HT + tetani (measured at 50–60 min after washout) in PFC neurons from stressed and control rats. *P < 0.005, ANOVA.
Discussion
In this study, we have revealed that serotonin facilitates the induction of LTD of glutamatergic synaptic transmission in PFC pyramidal neurons. Previously it has been found that in visual cortex or hippocampus, the LTP or LTD induced by tetanic stimuli can be regulated by serotonin (Kojic et al. 1997, 2000; Edagawa et al. 2000, 2001; Shakesby et al. 2002). Tetanic stimuli (50 Hz, 100 pulses × 4 in 10 s intervals) delivered to layer II–III fibres of PFC did not induce significant changes of glutamatergic transmission in deep layers (V–VI) of PFC pyramidal neurons, similar to what has been found before (Otani et al. 1998). However, the same electrical stimuli coupled to 5-HT application induced a long-lasting depression of the excitatory transmission, while 5-HT alone only transiently depressed the glutamatergic response, indicating that serotonin plays an important role in modulating synaptic plasticity in the PFC network. This effect is primarily mediated by 5-HT2A/C receptors. Consistently, these receptors have been implicated in the regulation of synaptic plasticity in visual cortex (Kojic et al. 1997, 2000; Edagawa et al. 2000) or hippocampus (Wang & Arvanov, 1998; Tecott et al. 1998).
Our results have shown that the serotonin-facilitated LTD in PFC neurons requires concurrent synaptic activation of group I metabotropic glutamate receptors during tetanus, but not NMDA receptors. Based on the amino acid sequences and signal transduction mechanisms, the eight mGluR subtypes are divided into three groups: group I mGluRs (mGluR1 and mGluR5) stimulate phospholipase C and phosphoinositide hydrolysis, group II (mGluR2 and mGluR3) and group III mGluRs (mGlu4, mGluR6–8) primarily couple to the inhibition of cAMP formation (Pin & Duvoisin, 1995). While group II and III mGluRs are largely regarded as presynaptic (Gereau & Conn, 1995; Petralia et al. 1996), group I mGluRs are abundantly expressed in postsynaptic elements throughout cortex (Romano et al. 1995), with mGluR5 localized on dendritic spines and shafts of cortical pyramidal neurons (Romano et al. 1995) and mGluR1 present in non-pyramidal cortical neurons (Fotuhi et al. 1993). Many forms of synaptic plasticity rely on mGluR-mediated signalling (Bashir et al. 1993; Cho & Bashir, 2002). Mice lacking mGluR subtypes show impaired learning and altered synaptic plasticity (Aiba et al. 1994; Yokoi et al. 1996; Lu et al. 1997). Thus, mGluRs have been implicated in regulating neuronal communication and signal processing underlying higher cognitive functions (Nakanishi, 1994; Conn & Pin, 1997). In this study, we found that the LTD induction by serotonin and tetanic stimulation was blocked by group I mGluR antagonists, and co-application of serotonin and the group I mGluR agonist, but neither drug alone induced LTD without tetani, suggesting that co-activation of 5-HT receptors and group I mGluRs is sufficient for LTD induction in PFC.
To identify the signalling molecules mediating the serotonin-facilitated LTD induction in PFC, we have examined MAP kinases, a family of serine/threonine protein kinases including the extracellular signal-regulated kinases (p42/44 ERK1/2), p38 MAPK and the c-Jun N-terminal kinases (JNKs 1/2/3). Both p42/44 MAPK and p38 MAPK have been implicated in various forms of synaptic plasticity (Bolshakov et al. 2000; Zhu et al. 2002; Rush et al. 2002; Guan et al. 2003). The serotonin + tetanus-induced LTD in PFC neurons was blocked by postsynaptic injection of a specific p38 MAPK inhibitor but not p42/44 MAPK inhibitors, as well as by intracellular dialysis with the antibody against activated p38 MAPK but not activated p42/44 MAPK, suggesting that p38 MAPK mediates the serotonin-facilitated LTD in PFC. Moreover, our biochemical data have shown that combined activation of 5-HT receptors and group I mGluRs synergistically increased the level of active (dual phosphorylated) p38 MAPK, further indicating that both receptors may cooperate to induce LTD through converging activation of postsynaptic p38 MAPK. It is different from dopamine-facilitated prefrontal cortical LTD, which is mediated by p42/44 MAPK activation (Otani et al. 1999). Consistent with our finding on the involvement of p38 MAPK in LTD of glutamatergic synapses in PFC neurons, p38 MAPK pathway has been found to mediate the induction of mGluR-dependent LTD in hippocampus, while p42/p44 MAPK pathway mediates LTP (Bolshakov et al. 2000; Zhu et al. 2002). The mechanism for p38 activation in response to co-stimulation of 5-HT2 receptors and group I mGluRs is not very clear. Presumably, the increase of [Ca2+]i triggered by 5-HT + tetanus activates the small GTPase Rap1 via a PKA-dependent mechanism (Grewal et al. 2000), which leads to the activation of its downstream target p38 MAPK (Sawada et al. 2001; Zhu et al. 2002; Huang et al. 2004).
One of the important downstream targets of p38 MAPK that is potentially involved in LTD is Rab5 (Cavalli et al. 2001; Huang et al. 2004), a member of the Rab family of small GTPases that function as specific regulators of vesicle transport between organelles (Zerial & McBride, 2001; Pfeffer, 2001). Rab5 is a key coordinator of early endocytic trafficking events including early endosome fusion (Gorvel et al. 1991), internalization (Bucci et al. 1992), clathrin-coated vesicle formation (McLauchlan et al. 1998), and motility of early endosomes on microtubules (Nielsen et al. 1999). In addition to cycling between inactive (GDP-bound) and active (GTP-bound) states, Rab5 also cycles between a membrane-bound and a cytosolic state depending on the guanyl-nucleotide dissociation inhibitor (GDI, Sasaki et al. 1990). Activation of p38 MAPK stimulates formation of the GDI : Rab5 complex (Cavalli et al. 2001; Huang et al. 2004), which leads to the delivery of Rab5 to the plasma membrane where it is activated and triggers endocytosis by facilitating the formation of clathrin-coated pits and sorting of membrane proteins into endosomes (Bucci et al. 1992; McLauchlan et al. 1998). Using antibody-mediated inactivation of membrane-associated Rab5 (Gorvel et al. 1991) or injection with the dominant negative Rab5 (Stenmark et al. 1994), the serotonin-facilitated LTD induction was blocked, suggesting that activation of Rab5 is required for the synaptic plasticity in PFC.
Given the key role of Rab5 in regulating protein transport from plasma membrane to early endosomes (Bucci et al. 1992; de Hoop et al. 1994), one possibility underlying the serotonin-facilitated depression of glutamatergic transmission is the Rab5-mediated AMPAR internalization (Huang et al. 2004; Brown et al. 2005). AMPAR endocytosis plays a critical role in hippocampal LTD (Beattie et al. 2000; Malinow & Malenka, 2002). Removal of synaptic AMPARs is mediated by clathrin-dependent endocytosis (Carroll et al. 1999; Man et al. 2000) through the interaction between the AMPA receptor GluR2 subunit and the clathrin adaptor protein AP2 (Lee et al. 2002). In the clathrin-mediated transport pathway, the GTPase dynamin is thought to be involved in ‘pinching off’ endocytic vesicles from the plasma membrane (Schmid et al. 1998). Injection of a dynamin inhibitory peptide, which interferes with the binding of amphiphysin with dynamin and therefore prevents endocytosis through clathrin-coated pits (Gout et al. 1993; Grabs et al. 1997), blocked the serotonin-facilitated LTD induction, suggesting that clathrin-dependent AMPAR endocytosis mediates the synaptic plasticity in PFC neurons.
Finally, we examined the functional implication of the serotonin-regulated LTD in PFC. We found that the serotonin-facilitated long-term plasticity of glutamatergic transmission in PFC is impaired in animals exposed to stress. Moreover, our previous study has shown that acute stress alters the serotonergic regulation of GABA transmission in PFC pyramidal neurons (Tan et al. 2004). These results provide a framework for understanding the interactions between monoamines, glutamate and GABA in normal mental functions and neuropsychiatric disorders (Carlsson et al. 2001).
Acknowledgments
This work was supported by NIH grants and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award to Z.Y. We would like to thank Xiaoqing Chen for her technical support.
References
- Abi-Dargham A, Laruelle M, Aghajanian GK, Charney D, Krystal J. The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci. 2000;3:1107–1112. doi: 10.1038/80624. [DOI] [PubMed] [Google Scholar]
- Breier A. Serotonin, schizophrenia and antipsychotic drug action. Schizophr Res. 1995;14:187–202. doi: 10.1016/0920-9964(94)00043-8. [DOI] [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Buhot MC. Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates NMDA receptor channel trafficking and function in cortical neurons. Mol Pharm. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- Cho K, Bashir ZI. Cooperation between mGluR receptors: a depressing mechanism? Trends Neurosci. 2002;25:405–411. doi: 10.1016/s0166-2236(02)02228-2. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;7:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Dubovsky SL, Thomas M. Serotonergic mechanisms and current and future psychiatric practice. J Clin Psychiatry. 1995;56(Suppl. 2):38–48. [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. The serotonin 5-HT2 receptor–phospholipase C system inhibits the induction of long-term potentiation in the rat visual cortex. Eur J Neurosci. 2000;12:1391–1396. doi: 10.1046/j.1460-9568.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001;21:1532–1537. doi: 10.1523/JNEUROSCI.21-05-01532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao JH, Yan Z. Serotonin receptors modulate GABAA receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RW, 4th, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Horgan AM, York RD, Withers GS, Banker GA, Stork PJ. Neuronal calcium activates a Rap1 and B-Raf signaling pathway via the cyclic adenosine monophosphate-dependent protein kinase. J Biol Chem. 2000;275:3722–3728. doi: 10.1074/jbc.275.5.3722. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yan Z. Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol Pharmacol. 2004;66:948–955. doi: 10.1124/mol.104.001404. [DOI] [PubMed] [Google Scholar]
- Gu Z, Zhong P, Yan Z. Activation of muscarinic receptors inhibits beta-amyloid peptide-induced signaling in cortical slices. J Biol Chem. 2003;278:17546–17556. doi: 10.1074/jbc.M209892200. [DOI] [PubMed] [Google Scholar]
- Guan Z, Kim JH, Lomvardas S, Holick K, Xu S, Kandel ER, Schwartz JH. p38 MAP kinase mediates both short-term and long-term synaptic depression in Aplysia. J Neurosci. 2003;23:7317–7325. doi: 10.1523/JNEUROSCI.23-19-07317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, You JL, Wu MY, Hsu KS. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J Biol Chem. 2004;279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- Ives JH, Fung S, Tiwari P, Payne HL, Thompson CL. Microtubule-associated protein light chain 2 is a stargazin-AMPA receptor complex-interacting protein in vivo. J Biol Chem. 2004;279:31002–31009. doi: 10.1074/jbc.M402214200. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia HK, Brisorgueil MJ, Hamon M, Calas A, Verge D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci U S A. 2000;97:1951–1952. doi: 10.1073/pnas.070044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci U S A. 2000;97:1841–1844. doi: 10.1073/pnas.97.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors – mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Martin GR, Eglen RM, Hamblin MW, Hoyer D, Yocca F. The structure and signalling properties of 5-HT receptors: an endless diversity? Trends Pharmacol Sci. 1998;19:2–4. doi: 10.1016/s0165-6147(97)01143-7. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Marzo A, Otani S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci. 2006;26:4803–4810. doi: 10.1523/JNEUROSCI.5312-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM. Does Stress Cause Psychiatric Illness? Washington, DC, USA: American Psychiatric Press; 1995. [Google Scholar]
- Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, Mori K, Togashi H, Ueno K, Kaku A, Yoshioka M. Changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949:131–138. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce JM, Crepel F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience. 1998;85:669–676. doi: 10.1016/s0306-4522(97)00677-5. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacol. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rush AM, Wu J, Rowan MJ, Anwyl R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J Neurosci. 2002;22:6121–6128. doi: 10.1523/JNEUROSCI.22-14-06121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- Sawada Y, Nakamura K, Doi K, Takeda K, Tobiume K, Saitoh M, Morita K, Komuro I, De Vos K, Sheetz M, Ichijo H. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci. 2001;114:1221–1227. doi: 10.1242/jcs.114.6.1221. [DOI] [PubMed] [Google Scholar]
- Schmid SL, McNiven MA, De Camilli P. Dynamin and its partners: a progress report. Curr Opin Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1991;1:113–120. doi: 10.1016/0959-4388(91)90018-3. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA. Neurobiology of serotonin in depression and suicide. Ann N Y Acad Sci. 1997;836:220–232. doi: 10.1111/j.1749-6632.1997.tb52362.x. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. [Google Scholar]
- Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci. 2004;24:5000–5008. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Logue SF, Wehner JM, Kauer JA. Perturbed dentate gyrus function in serotonin 5-HT2C receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15026–15031. doi: 10.1073/pnas.95.25.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Arvanov VL. M100907, a highly selective 5-HT2A receptor antagonist and a potential atypical antipsychotic drug, facilitates induction of long-term potentiation in area CA1 of the rat hippocampal slice. Brain Res. 1998;779:309–313. doi: 10.1016/s0006-8993(97)01174-8. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Yan Z. Regulation of GABAergic inhibition by serotonin signaling in prefrontal cortex: molecular mechanisms and functional implications. Mol Neurobiol. 2002;26:203–216. doi: 10.1385/MN:26:2-3:203. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhong P, Gu Z, Wang X, Jiang H, Feng J, Yan Z. Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer's disease. J Biol Chem. 2003;278:26888–26896. doi: 10.1074/jbc.M302789200. [DOI] [PubMed] [Google Scholar]
- Zhong P, Yan Z. Chronic antidepressant treatment alters serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. Neuroscience. 2004;129:65–73. doi: 10.1016/j.neuroscience.2004.06.072. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]