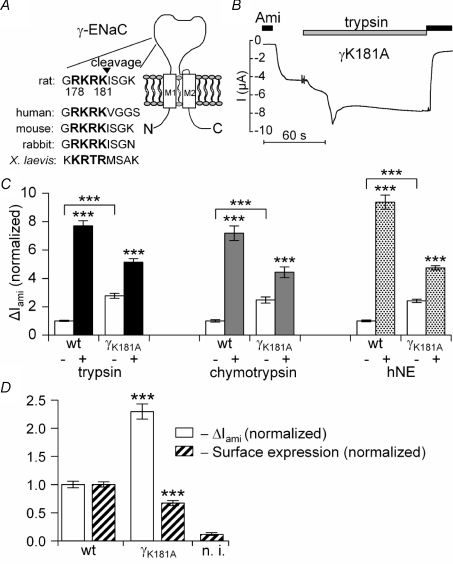
Figure 3. Mutating a putative prostasin site in the γ-subunit increases ENaC whole-oocyte currents, but decreases channel surface expression and reduces the stimulatory effect of extracellular proteases.
In A, the amino acid sequence alignment illustrates a highly conserved putative prostasin cleavage site (bold letters) in γ-ENaC of different species. In B and C, αβγ-ENaC (wt) or αβ-ENaC together with the mutant K181A γ-ENaC (γK181A) were expressed in Xenopus laevis oocytes to test the effect of the mutation on ΔIami baseline whole-oocyte currents and on the responsiveness to extracellular proteases. A representative whole-oocyte current (I) trace from an oocyte expressing γK181A is shown in B. In C, open columns represent the average ΔIami values before application of proteases. The black, grey and dotted columns represent the average ΔIami values 2 min after exposure to trypsin (2 μg ml−1; n= 82 for wt, n= 83 for γK181A; N= 8), chymotrypsin (2 μg ml−1; n= 43 for each group; N= 3) or human neutrophil elastase (hNE) (10 μg ml−1; n= 32 for each group; N= 3), respectively. ΔIami values were normalized to the average ΔIami measured in the αβγ-ENaC expressing oocytes before the application of proteases. In D, surface expression (hatched columns) and ΔIami (open columns) were measured in matched oocytes expressing either wild-type ENaC or the γK181A mutant channel (N= 4). Non-injected oocytes (n.i.) served as control.