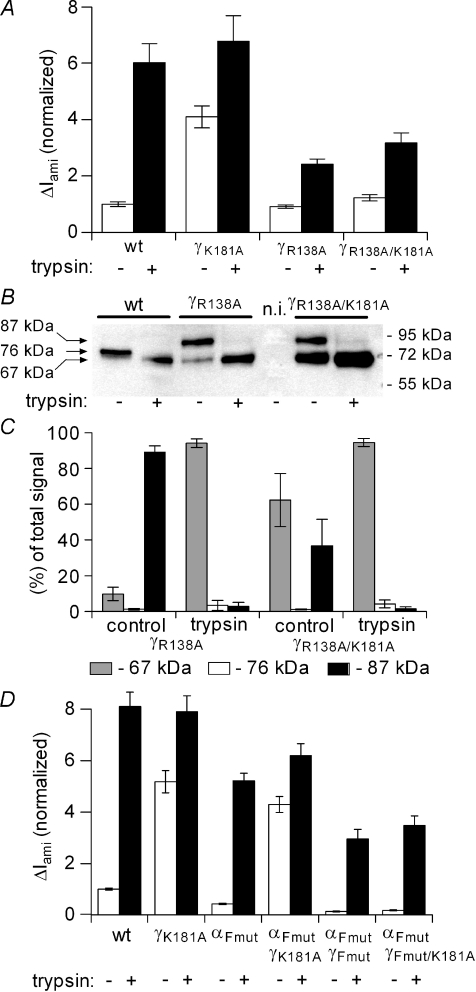
Figure 12. γR138A mutation prevents the gain-of-function phenotype of the γK181A mutation.
The experiments were essentially performed as described in Fig. 10 using oocytes expressing the C-terminal V5 tag constructs for wild-type γ-ENaC (wt), γK181A, γR138A or γR138R/K181A mutant subunits (γK181A, γR138A, γR138A/K181A, respectively). A, average ΔIami values are shown for wt, γK181A, γR138A and γR138A/K181A expressing oocytes before (open columns; trypsin –) and after (filled columns, trypsin +) exposure to trypsin (2 μg ml−1) for 2 min. ΔIami values were normalized to the average ΔIami measured in wt-expressing oocytes from the same batch prior to the application of trypsin (for each column: n= 24; N= 3). B, the lanes of the representative Western blot correspond to the experimental conditions of the current data shown in A. C, summary of Western blot results for γR138A- and γR138A/K181A-expressing oocytes from similar experiments (n= 3; N= 3) as shown in B. Average ΔIami values shown in D were obtained from oocytes expressing either wt-αβγ-ENaC (wt) or channels with one or two mutated subunits as indicated below the columns: γK181A, αFmut (αR205A/R231A), γFmut (γR138A) or γFmut/K181A (n= 18; N= 3 for each group).