Abstract
Transplantation of mouse embryonic stem (mES) cells can restore function in Parkinson disease models, but can generate teratomas. Purification of dopamine neurons derived from embryonic stem cells by fluorescence-activated cell sorting (FACS) could provide a functional cell population for transplantation while eliminating the risk of teratoma formation. Here we used the tyrosine hydroxylase (TH) promoter to drive enhanced green fluorescent protein (eGFP) expression in mES cells. First, we evaluated 2.5-kilobase (kb) and 9-kb TH promoter fragments and showed that clones generated using the 9-kb fragment produced significantly more eGFP+/TH+ neurons. We selected the 9-kb TH clone with the highest eGFP/TH overlap for further differentiation, FACS, and transplantation experiments. Grafts contained large numbers of eGFP+ dopamine neurons of an appropriate phenotype. However, there were also numerous eGFP+ cells that did not express TH and did not have a neuronal morphology. In addition, we found cells in the grafts representing all three germ layers. Based on these findings, we examined the expression of stem cell markers in our eGFP+ population. We found that a majority of eGFP+ cells were stage-specific embryonic antigen-positive (SSEA-1+) and that the genetically engineered clones contained more SSEA-1+ cells after differentiation than the original D3 mES cells. By negative selection of SSEA-1, we could isolate a neuronal eGFP+ population of high purity. These results illustrate the complexity of using genetic selection to purify mES cell-derived dopamine neurons and provide a comprehensive analysis of cell selection strategies based on tyrosine hydroxylase expression.
Keywords: Genetic engineering, Fluorescence-activated cell sorting, Parkinson disease, Stage-specific embryonic antigen 1, CD-15
Introduction
Embryonic stem (ES) cells, transplanted either in a naïve or a predifferentiated state, can alleviate symptoms in animal models of Parkinson disease [1-4]. However, transplantation of undifferentiated as well as in vitro differentiated ES cells can result in aberrant growth and tumor or teratoma formation [1, 5, 6]. Genetic purification of neural precursors using fluorescence-activated cell sorting (FACS) for Sox1+ cells can efficiently remove unwanted proliferating cells and subsequently avoid teratoma formation, but the number of dopamine neurons generated from such Sox1+ cells is low [7, 8]. Transplantation of mouse embryonic stem (mES) cell-derived dopamine neurons, purified by FACS, would, however, both eliminate possible teratoma formation and potentially improve behavioral recovery in a Parkinson disease model, compared with a mixed cell population. Earlier studies using either surface marker expression [9], dye labeling [10], or transgenic expression of enhanced green fluorescent protein (eGFP) from the rat [11] or human [12] tyrosine hydroxylase (TH) promoters have indicated that primary dopamine neurons can be purified from embryos by FACS and survive replating into culture and transplantation.
Transgenic mouse experiments using the rat TH promoter have indicated that 5′ sequences ≥4.5 kilobases (kb) [11, 13-20] in length are sufficient to drive TH expression to most catecholaminergic neurons in vivo. The use of shorter TH promoter fragments has yielded conflicting results, with ≤2.5 kb of 5′ sequence shown to be either insufficient to drive TH expression to cathecholaminergic neurons [17, 21] or sufficient to drive expression of TH specifically to midbrain dopamine neurons [16]. A 3.6-kb fragment could activate the TH gene, although the expression was not specific to catecholaminergic neurons [22]. The phenomenon of reporter gene expression in noncatecholaminergic neurons has been more prevalent with the use of shorter 5′ sequences, but it has also been observed to some extent when longer 5′ sequences have been used [15-17, 19, 20, 22]. In our strategy to purify dopamine neurons from mES cells, we compared the ability of a 2.5-kb fragment and a 9-kb fragment of the rat TH promoter to drive eGFP expression in mES cells after in vitro differentiation. Since the integration site could affect the promoter function, we generated multiple mES clones and analyzed their differentiation in vitro by immunofluorescent staining and FACS. One clone was finally selected based on its high overlap in eGFP and TH expression and further characterized in vitro as well as in vivo after FACS and transplantation into naïve mice and 6-hydroxydopaminelesioned rats.
Materials and Methods
Construction of 2.5-kb TH and 9-kb TH Promoter Plasmids
The 2.5- or 9.0-kb promoter region of the rat TH gene [23] (National Center for Biotechnology Information accession no. AF014956) were inserted into the multiple cloning site of the pEGFP-1 promoterless vector (GenBank accession no. U55761; Clontech, Palo Alto, CA, http://www.clontech.com), upstream of the eGFP gene. The 2.5-kb TH promoter fragment was retrieved by digestion with SfiI, and the 9-kb TH promoter fragment was retrieved by digestion with HindIII. Insert orientation was verified by double diagnostic digestion and sequence analysis of boundaries.
mES Cell Propagation
The mouse blastocyst-derived embryonic stem cell line D3 (ATCC, Manassas, VA, http://www.atcc.org) was propagated on mitomycin C-treated (10 μg/ml medium; Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) primary murine embryonic fibroblasts (PMEFs) (no. 00321; Stem Cell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 2 mM L-glutamine (Invitrogen), 1 mM β-mercaptoethanol, 1× nonessential amino acids (Invitrogen), 1× nucleo-sides (Specialty Media; Chemicon, Temecula, CA, http://www. chemicon.com), 15% fetal bovine serum (FBS) (Sigma-Aldrich), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), and 2,000 U/ml human recombinant leukemia inhibitory factor (R&D Systems Inc., Minneapolis, http://www.rndsystems.com). D3 cells were passaged four times before transfection and were subsequently purified from PMEFs.
Stable Transfection, Clonal Expansion, and Differentiation of mES Cells
D3 mES cells were transfected with 2.5-kb TH-pEGFP-1, 9-kb TH-pEGFP-1, or pEGFP-1 alone using Lipofectamine plus (Invitrogen). Stably transfected cells were selected in ES medium containing 500 μg/ml neomycin (Clontech). We screened a large number of independent drug-resistant colonies (108 of the smaller-sized colonies of each construct) and aimed to isolate those exhibiting a faithful co-expression pattern of TH and eGFP after in vitro differentiation on PA6 (Riken, Tsukuba, Japan, http//:www.riken.jp) [24]. A few empty vector clones were selected as negative controls for eGFP expression. Based on eGFP/TH overlap after differentiation on PA6 for 14 days, two clones of the 2.5-kb TH-pEGFP-1 (2.5-kb TH-eGFP) and three clones of the 9-kb TH-pEGFP-1 (9-kb TH-eGFP) construct mES cells were selected for expansion. These stably transfected clones were subsequently expanded on a mitomycin-treated, G418-resistant STO mouse embryonic fibroblast cell line, produced by transfection of regular STO cells (ATCC) with the pEGFP-1 empty vector and selection of neomycin-resistant cells. Next, expanded clones were seeded at 500-1,000 cells per cm2 onto mitomycin-treated PA6 or MS5 feeders and differentiated for 9-14 days [3, 24] (supplemental online data), to compare which protocol would generate appropriate eGFP+ neurons for transplantation. Prior to in vitro differentiation, mES cell were purified from STO feeders. Cells used for immunofluorescent staining were fixed in 4% paraformaldehyde for 30 minutes and rinsed with phosphate-buffered saline (PBS). Cells to be used for FACS and transplantation were harvested at days 8-11 of differentiation using 0.05% trypsin/EDTA. For both PA6- and MS5-based protocols, the full differentiation time is 14 days. Cells to be further analyzed in vitro after FACS were plated onto primary rat astrocytes (Cambrex, Walkersville, MD, http://www.cambrex.com) and supplemented with 10 ng/ml glial-derived neurotrophic factor (GDNF) and 20 ng/ml brain-derived neurotrophic factor (BDNF).
FACS
Cells were differentiated for 8-11 days, harvested using 0.05% Trypsin/EDTA (Invitrogen), gently dissociated into a single-cell suspension, and resuspended in phenol-free Hanks' balanced salt solution (Invitrogen) containing 20 mM glucose (Sigma-Aldrich), penicillin-streptomycin, and 2% FBS. Samples were filtered, analyzed, and sorted immediately or were subjected to surface marker staining: mouse anti-stage-specific embryonic antigen-positive (anti-SSEA-1) antibody (0.4 μg/ml; Developmental Studies Hybridoma Bank, Iowa City, IA, http://www.uiowa.edu/~dshbwww), incubated for 50 minutes at 4°C, washed, and then incubated with the corresponding secondary antibody followed by washing steps. Cells were analyzed and sorted using a FACSAria cell sorter and FACSDiva software (BD Biosciences, San Diego, http://www.bdbiosciences.com). The population of interest was identified by forward and side scatter gating. Using a 488-nm laser for excitation, GFP positivity was determined according to fluorescence intensity in the GFP channel (490-nm long-pass [LP], 510/20-nm band-pass [BP] filters) against autofluorescence in the yellow fluorescent protein (527 LP, 550/30 BP) or red (595 LP, 610/20 BP) channels. D3 mES cells or EV-1 cells, at the same stage of differentiation, were used as the GFP-negative controls. eGFP positivity was confirmed by reanalysis by FACS, and viability was determined by trypan blue exclusion. SSEA-1 positivity was determined according to fluorescence in the red channel compared with negative controls lacking the primary antibody and/or secondary antibodies. Further flow cytometric analysis was performed using FlowJo software (Tree Star, Ashland, OR, http://www.treestar.com).
Animal Procedures
All animal procedures were performed in accordance with National Institutes of Health guidelines and were approved by the Animal Institutional Care and Use Committee at McLean Hospital, Harvard Medical School.
Transplantation of eGFP+ Cells to Naïve Mice and 6-Hydroxydopamine-Lesioned Rats
Nine-kb TH-eGFP mES cells, FACS purified for eGFP+, were resuspended at 100,000 cells per microliter. Female naïve C57/Bl6 mice (n = 15) were grafted with 1-2 μl of cell suspension, and Sprague-Dawley rats with unilateral 6-hydroxydopamine lesions (Charles River Laboratories, Wilmington, MA, http://www.criver.com) were grafted with 3 μl of cell suspension (n = 18) or medium alone (n = 7). Transplantations and immunosuppression were performed as previously described [1]. Rotational behavior of the 6-hydroxydopamine-lesioned rats in response to amphetamine was measured as previously described [1] (supplemental online data). Mice were sacrificed 4 weeks and rats 10 weeks after transplantation, unless needed earlier. Animals were anesthetized by an i.p. overdose of pentobarbital (150 mg/kg) and perfused intracardially with heparinized saline (0.1% heparin) followed by 4% paraformaldehyde. Brains were removed, postfixed for 6 hours in paraformaldehyde, equilibrated in 20% sucrose, and sectioned on a freezing microtome in 40-μm serially collected coronal slices.
Histological and Stereological Procedures
For immunofluorescent staining, cells/sections were incubated with blocking buffer (PBS, 10% normal donkey serum, 0.1% Triton X-100) for 1 hour and subsequently with primary antibodies in blocking buffer overnight. Information on primary antibodies used is given in supplemental online data. The coverslips/sections were subsequently incubated in fluorescent-labeled secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) in blocking buffer for 1 hour, rinsed in PBS, counterstained with Hoechst 33342 (4 μg/ml), and mounted (Gel/Mount; Biomeda Corp., Foster City, CA, http://www.biomeda.com). For light microscopy, a biotinylated secondary antibody (1:300; Vector Laboratories, Burlingame, CA, http://www. vectorlabs.com) was used, followed by incubation in streptavidin-biotin complex (Vector Laboratories) and 3,3′-diaminobenzidine (Vector Laboratories). Confocal analysis was performed using a Zeiss LSM510/Meta Station (Thornwood, NY, http://www.zeiss.com). Stereology was performed using Stereo Investigator software (MicroBrightField, Williston, VT, http://www.microbrightfield.com) and a Zeiss Axioplan I fluorescent microscope. Graft volumes and TH neuron numbers were calculated using the Cavalieri estimator and Optical fractionator probes. The eGFP/TuJ1 and eGFP/TH overlap in cells after in vitro differentiation (≥3 cover-slips per condition) and the percentage of eGFP+ or eGFP- TH+ cells in the grafts (n = 6) was determined by random sampling using StereoInvestigator. eGFP and TH overlap in purified eGFP+/SSEA-1- neurons was assessed by randomized quantification of TH expression (647 nm) in cells identified as eGFP+ (488 nm) using confocal microscopy (three coverslips were counted).
Statistical Analysis
The number of eGFP+ events in TH-GFP clones versus the original D3 mES clone, as well as the rotations for mES cell-grafted and vehicle-grafted animals, was analyzed using analysis of variance (ANOVA). The extent of overlap between eGFP and TuJ1 or TH after in vitro differentiation of 9-kb TH-eGFP cells was determined by unpaired t test. The percentage of TH+ cells in the rat grafts that were eGFP+ or eGFP- was analyzed by paired t test. InStat3 software (GraphPad Software, Sa Diego, http://www.graphpad.com) was used for all statistical analyses.
Results
Evaluation of TH-eGFP Promoter Constructs in mES Cells In Vitro
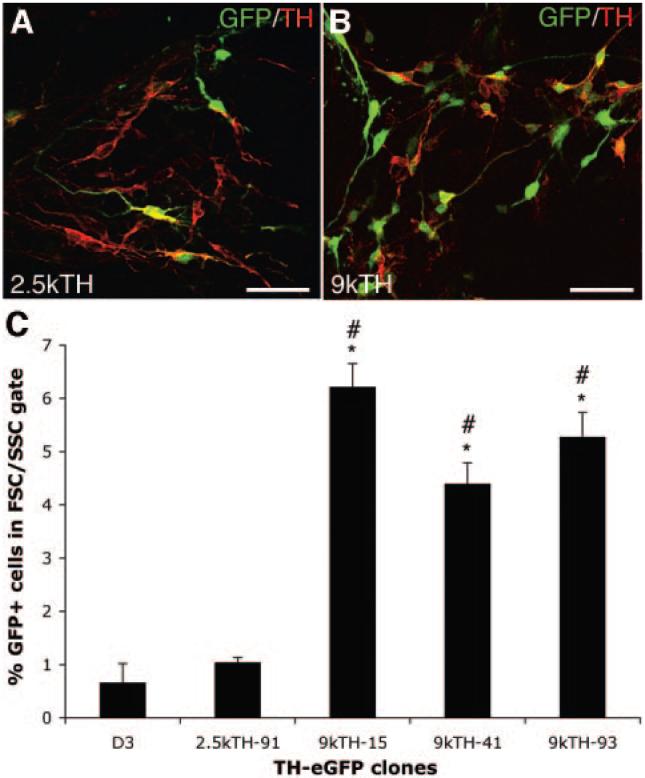
We transfected naïve D3 mES cells with a 2.5-kb (2.5-kb TH-eGFP) or a 9-kb (9-kb TH-eGFP) rat TH promoter construct driving eGFP expression. G418-resistant clones were screened for overlap between eGFP and TH expression after 14 days of differentiation using the PA6-based protocol [24]. Based on previous TH promoter studies, the 2.5-kb TH promoter construct was expected to either generate eGFP+/TH+ neurons of a midbrain fate only, or to be insufficient to drive eGFP expression to dopamine neurons. The 9-kb TH promoter construct was expected to generate large numbers of eGFP+/TH+ neurons of all TH phenotypes. However, the cell culture conditions used appear to enrich for midbrain dopamine neurons and minimize the generation of norepinephric neurons or non-neuronal TH cells as seen after transplantation [3, 6]. Screening of 108 clones of each construct after differentiation by TH and eGFP immunofluorescence showed that only 10 of 108 of the 2.5-kb TH-GFP clones generated eGFP+ neurons, and of these, only two clones showed any overlap with TH expression (Fig. 1A). However, also in these two 2.5-kb TH-eGFP clones, most eGFP+ cells generated were not overlapping with TH expression and many of the eGFP+ cells appeared to be of a non-neuronal morphology (data not shown; Fig. 1A). In contrast, 85 of 108 of the 9-kb TH-eGFP clones generated eGFP+ neurons after differentiation, and >10 of these clones generated eGFP+ neurons with overlapping TH expression (Fig. 1B). FACS analysis was used to further characterize the clones (three 9-kb TH-eGFP and one 2.5-kb TH-eGFP) showing the highest level of eGFP/TH co-expression in vitro. FACS for eGFP at days 8, 9, 10, and 11 of in vitro differentiation indicated that day 9 was the optimal time point for sorting of the cells. eGFP+ cells generated after 9 days in culture could survive the dissociation and FACS procedures, whereas at later time points, survival was lower (data not shown). FACS analysis showed that all 9-kb clones tested contained significantly higher numbers of eGFP+ cells 6.2% ± 0.44% (n = 3), 4.4% ± 0.39% (n = 3), and 5.28% ± 0.46% (n = 3) (Fig. 1C) compared with the naïve D3 mES cells (0.66% ± 0.36% [n = 3]; p < .05 to p < .001, ANOVA) and the 2.5-kb TH-eGFP clone (1.04% ± 0.09% [n = 3]; p < .01 to p < .001, ANOVA). The 2.5-kb TH-eGFP clone was not significantly different from the naïve D3 mES cells (Fig. 1C). Consequently, we selected one of the 9-kb clones (9kTH-15) showing high co-expression of TH and eGFP (Fig. 1B, 1C), for the remaining experiments.
Figure 1.
Evaluation of TH-eGFP promoter constructs in mouse embryonic stem (mES) cells during in vitro differentiation. (A, B): Overlap in eGFP and TH expression in a (A) 2.5-kilobase (kb) TH-GFP clone and in a (B) 9-kb TH-eGFP clone after 14 days of differentiation on PA6. Cultures of 9-kb TH-eGFP clones contained many more eGFP+/TH+ cells compared with the 2.5-kb TH-GFP cultures (yellow co-expression). (C): FACS analysis for eGFP+ events within an FSC/SSC gate of naïve D3 mES cells autofluorescence), one 2.5-kb TH-eGFP clone (number 91) and three 9-kb TH-eGFP clones (numbers 15, 41, and 93) after 9 days of differentiation on PA6. All 9-kb TH clones showed an expected higher proportion of eGFP+ events compared with the naïve D3 mES cell line (*, p < .01 to p < .001; analysis of variance [ANOVA]) and to the 2.5-kb clone (#, p < .01 to p < .001; ANOVA). The 2.5-kb TH-eGFP clone was not significantly different from the D3 cells (*, p < .05 to p < .001; ANOVA). Scale bars = 50 μm (A, B). Abbreviations: eGFP, enhanced green fluorescent protein; FSC, forward scatter; GFP, green fluorescent protein; k, kilobase; SSC, side scatter; TH, tyrosine hydroxylase.
Comparison of MS5- and PA6-Based In Vitro Differentiation Protocols
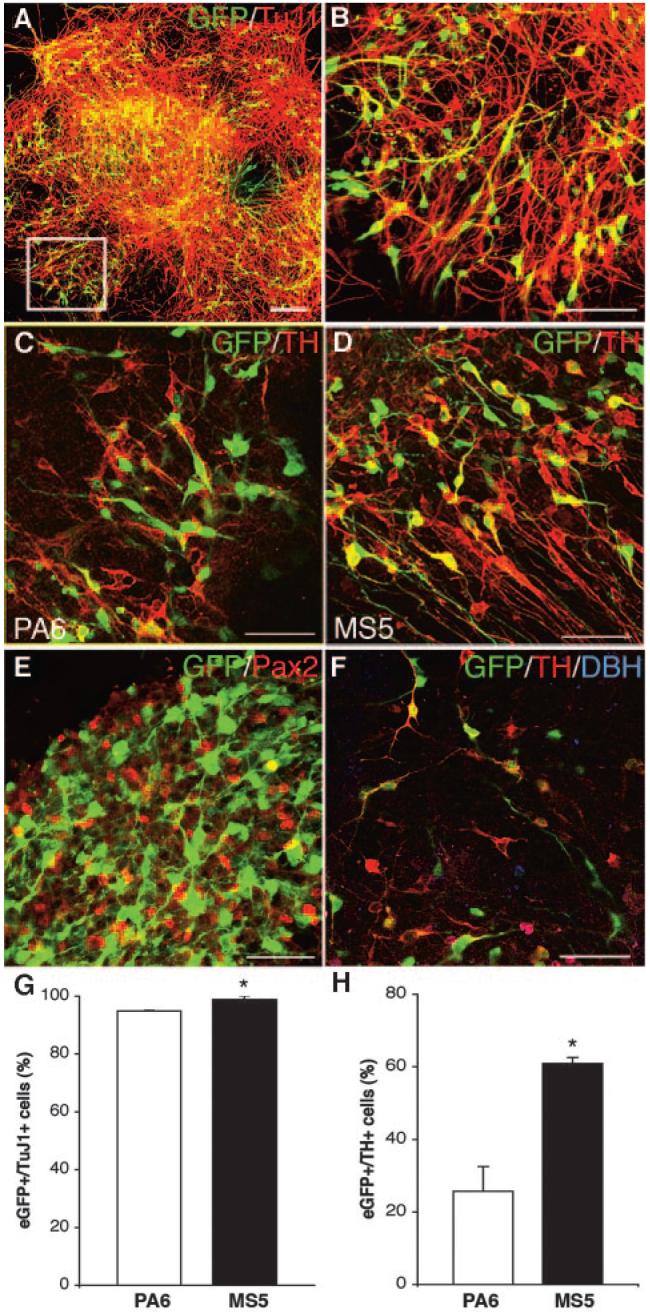
To evaluate which differentiation protocol would better generate appropriate eGFP+ neurons for transplantation, the 9-kb TH-eGFP clone 15 (9-kb TH-eGFP) was differentiated using the PA6-based [24] and the MS5-based [3] protocols. In addition to generating the highest number of eGFP+ events by FACS (Fig. 1C), this 9-kb TH promoter clone also displayed the highest eGFP+/TH+ overlap of all clones (data not shown). An initial analysis using the PA6-based protocol showed that the eGFP+/TH+ overlap was similar at 9 and 14 days of differentiation (data not shown). Since FACS was optimally run at day 9 of differentiation, we chose this time point for all further in vitro analyses of our cells. Both PA6 and MS5 protocols generated large amounts of cells co-expressing eGFP and TuJ1, with the number of eGFP+/TuJ1+ cells being significantly higher on MS5 (98% ± 0.7%) compared to PA6 (94.9% ± 0.2%; p < .01, unpaired t test) (Fig. 2A, 2B, 2G). Furthermore, the proportion of eGFP+/TH+ cells was significantly higher using the MS5-based protocol (60.9% ± 1.6%) compared with the PA6-based protocol (25.7% ± 6.8%; p < .01, unpaired t test) (Fig. 2C, 2D, 2H). Immunofluorescent analysis showed abundant Pax2 expression, demonstrating the midbrain/hindbrain character of the culture system. The overlap between eGFP and Pax2 was minimal, as expected based on the different temporal expression of these genes [25-27] (Fig. 2E). Both coculture systems generated few dopamine β-hydroxylase-positive cells (Fig. 2F), and no eGFP+ cells had a norepinephric phenotype (Fig. 2F). Based on these findings, we used the MS5-based protocol in all subsequent experiments.
Figure 2.
Comparison of MS5- and PA6-based in vitro differentiation protocols. (A-H): The 9-kilobase (kb) TH-eGFP mouse embryonic stem cells were analyzed in vitro after 9 days of differentiation using either the PA6 or MS5-based protocols. (A), enlarged in (B): eGFP and TuJ1 overlap was high using either protocol, although it was significantly higher using the MS5-based protocol (PA6, 94.9% ± 0.2% [SEM]; MS5, 98.9% ± 0.7% [SEM]) (yellow co-expression) (G). eGFP and TH overlap was significantly higher using the MS5-based compared with the PA6-based protocol (C, D, G) (PA6, 25.7% ± 6.8% [SEM]; MS5, 60.9% ± 1.6% [SEM]) (yellow co-expression). Pax2 immunofluorescent staining (E) showed the mid-hindbrain characteristic of the MS5 culture, with very little overlap between eGFP and Pax2, as anticipated based on the different temporal expression of TH and Pax2 during normal development. (F): There were few DBH+ cells generated in either PA6- or MS5-based protocols, and the DBH staining did not appear to overlap with eGFP expression. Bar graphs depicting overlap between GFP and TuJ1 expression (G) and eGFP and TH expression (H) in cells differentiated using PA6-based (white bars) or MS5-based (black bars) protocol for 9 days (*, p < .01, unpaired t test). Scale bars = 100 μm (A) and 50 μm (B-F). Abbreviations: DBH, dopamine β-hydroxylase; eGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; Pax2, paired box gene 2; TH, tyrosine hydroxylase; TuJ1, class III β-tubulin.
Transplantation and In Vivo Analysis of eGFP-Sorted Cells
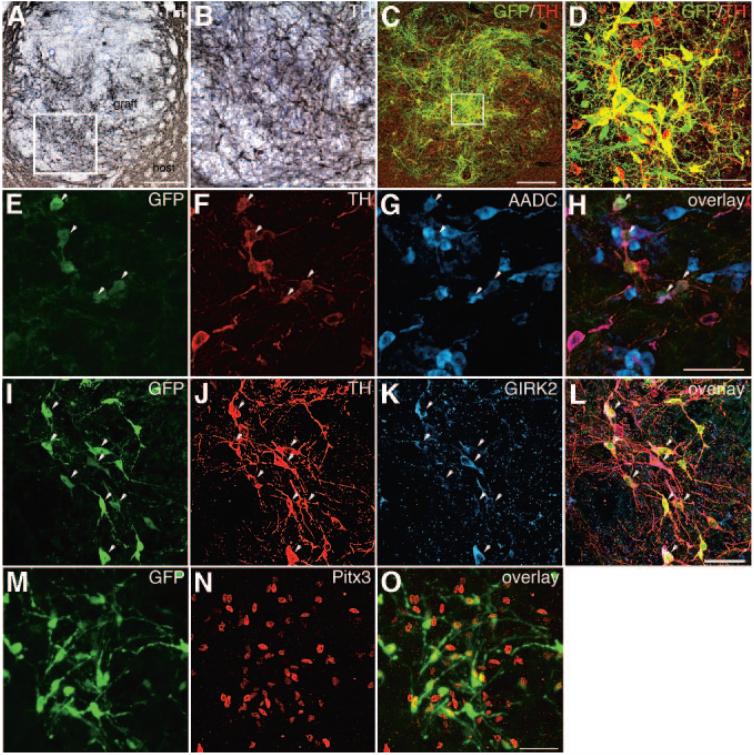
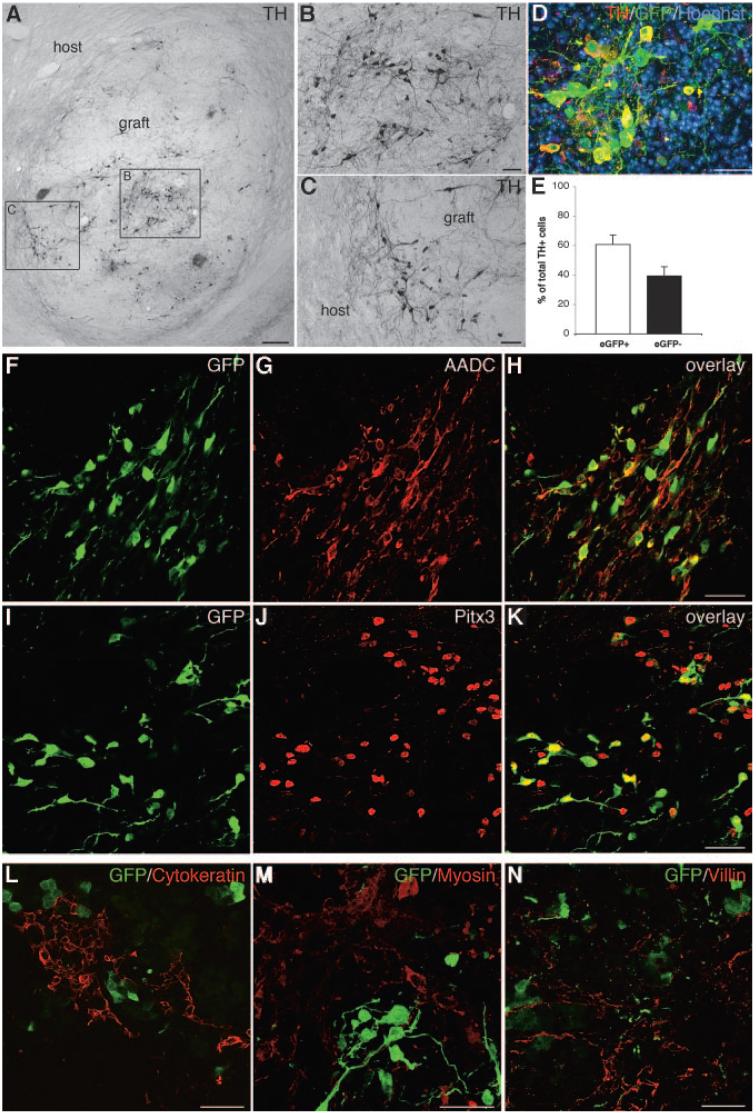
In vitro differentiated 9-kb TH-eGFP cells were FACS sorted for eGFP expression and transplanted. The short-term transplantation to mice showed the presence of eGFP+ cells and large numbers of TH neurons in all grafts (Fig. 3A-3D). Most TH+ neurons appeared to co-express eGFP (Fig. 3C, 3D). However, some cells expressed only eGFP or only TH (Fig. 3C, 3D). Further analysis of the dopamine phenotype of the eGFP+ neurons showed that many eGFP+ cells expressed aromatic L-amino acid decarboxylase (AADC) (Fig. 3E-3H), as well as GIRK2 (Fig. 3I-3L), which is relatively enriched in A9 compared with other midbrain dopamine neurons [28-30] (Fig. 3E-3H). In addition, most eGFP+ cells expressed the nuclear midbrain dopamine marker Pitx3 [31, 32] (Fig. 3M-3O). Unexpectedly, during the long-term transplantation of eGFP+ cells to 6-hydroxydopamine-lesioned rats, a number of animals started displaying behavioral deficits suggesting teratoma formation and were therefore perfused before the behavioral study was completed. Only animals surviving the entire 10 weeks of testing were included in the behavioral analysis (n = 8). When the number of rotations was analyzed, there was no significant difference between the vehicle and the cell-transplanted groups at any time point (p > .05, ANOVA; supplemental online Fig. 1A). The eight cell-grafted animals that survived until the 10-week time point had a 21% average reduction in the number of rotations (supplemental online Fig. 1B). Graft analysis in four of the surviving animals with reduced number of rotations showed the presence of large numbers of TH+ neurons: 12,567, 92,945, 102,515, and 165,326, respectively (supplemental online Fig. 1B). These numbers were directly correlated with the size of the grafts (data not shown) but not with the improvement in the number of rotations. The animal with the largest number of TH+ neurons (Fig. 4A-4E) had a 70% decrease in the number of rotations (supplemental online Fig. 1B), and this graft contained some TH+ neurons, which extended neurites into the host striatum (Fig. 4A, 4C). Approximately 60% of the TH+ neurons were also eGFP+ (Fig. 4D, 4E). The grafts also contained cells of neuronal morphology, which were either eGFP+/TH- or eGFP-/TH+ (Fig. 4D). Further analysis of the dopamine phenotype of the grafted cells showed that most of the eGFP+ neurons expressed AADC (Fig. 4F-4H) and Pitx3 (Fig. 4I-4K). All three germ layers, as visualized by cytokeratin (ectoderm), myosin (mesoderm), and villin (endoderm) staining, were present in the grafts (Fig. 4L-4N). No colocalization between eGFP and the germ layer markers was identified.
Figure 3.
Short-term transplantation and in vivo analysis of eGFP-sorted cells. (A-O): naïve mice were transplanted with cells sorted for eGFP expression. Grafts were analyzed 4 weeks post-transplantation. (A): Low-power microphotograph of a graft, showing large numbers of TH+ neurons (the nickel-enhanced 3,3′-diaminobenzidine products appear grayish black; the boxed area is shown enlarged in [B]). Most TH+ neurons appeared to co-express eGFP (C) (co-expression appears yellow; the boxed area is enlarged in [D]); however, some cells expressed only eGFP or only TH. A large proportion of the eGFP+/TH cells also showed overlapping expression with AADC (arrowheads) (co-expression appears purple) (E-H). Many eGFP+/TH+ cells showed overlapping GIRK2 immunoreactivity (arrowheads) (co-expression appears purple), but not all (I-L). Pitx3 was expressed in the nucleus of most eGFP+ cells (M-O). Scale bars = 150 μm (A), 30 μm (B), 100 μm (C), and 50 μm (D, H, L, O) (scale bar in [H] applies to [E-G], [L] applies to [I-K], and [O] applies to [M, N]). Abbreviations: AADC, aromatic L-amino acid decarboxylase; GFP, green fluorescent protein; GIRK2, G protein-activated inwardly rectifying potassium channel 2; Pitx3, paired-like homeodomain transcription factor 3; TH, tyrosine hydroxylase.
Figure 4.
Long-term xenotransplantation and in vivo analysis of eGFP-sorted cells. (A-N): eGFP+ cell transplants into 6-hydroxydopamine-lesioned rats were analyzed 10 weeks post-transplantation. (A): Low-power microphotograph of a graft, showing large numbers of TH+ neurons (the nickel-enhanced DAB products appear grayish black; the boxed area to the left is shown enlarged in [B], and the boxed area to the right is shown enlarged in [C]). Some of the grafted TH neurons extend neurites into the host striatum (C). (D): Many TH+ neurons were eGFP+ (co-expression appears yellow). (E): Bar graph depicting the percentage of total TH+ cells that were eGFP+ (white bar; 60.7% ± 6.4% [SEM]) or eGFP- (black bar; 39.3% ± 6.3% [SEM]). The two groups were not significantly different (p > .05, paired t test) due to the high variability in overlap between TH and eGFP between different grafts. Many of the eGFP+ neurons displayed characteristics of midbrain dopamine neurons, as shown by the overlapping expression with AADC (F-H) and Pitx3 (I-K). Grafts contained all three germ layers, as visualized by staining for cytokeratin (L), myosin (M), and villin (N), but no overlapping expression with eGFP was detected. Scale bars = 200 μm (A), 50 μm (B, C, F-N), and 25 μm (D). Abbreviations: AADC, aromatic L-amino acid decarboxylase; eGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; Pitx3, paired-like homeodomain transcription factor 3; TH, tyrosine hydroxylase.
Identification of Proliferative eGFP+ Cells in the Grafts
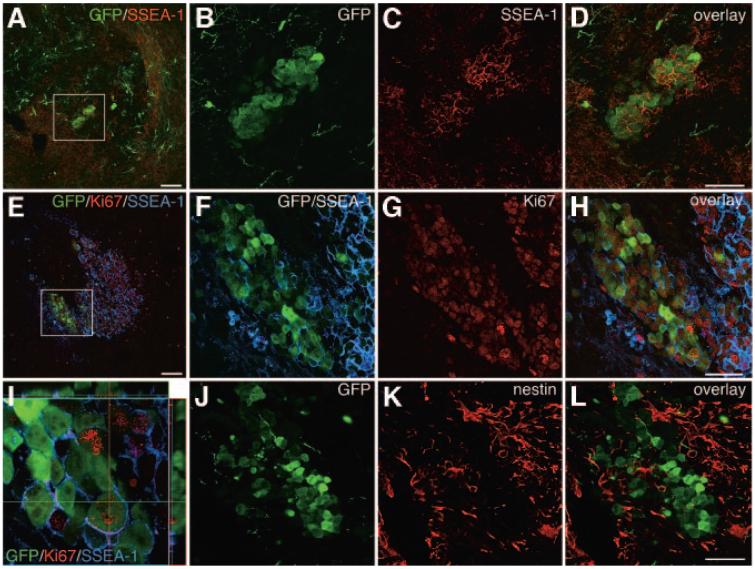
Further analysis showed that in addition to eGFP+ cells of a neuronal phenotype, grafts contained clusters of immature eGFP+/SSEA-1+ cells (Fig. 5A-5I). All grafts analyzed contained proliferative cells, as demonstrated by Ki67 staining (Fig. 5E-5I). The majority of Ki67+ cells in the grafts were not eGFP+. However, a large proportion of the eGFP+ cells showing a non-neuronal morphology were Ki67+ and some of these eGFP+/Ki67+ cells also expressed SSEA-1 on their surface (Fig. 5E-5I). There were large nestin+ areas in the grafts surrounding the GFP+ cells of immature morphology, but very few, if any, cells were GFP+/nestin+ (Fig. 5J-5L).
Figure 5.
Identification of proliferative eGFP+ cells in the grafts. (A-L): Analysis of mouse grafts 4 weeks post-transplantation showed that there were cluster of SSEA-1+/eGFP+ cells in some grafts ([A]; the boxed area is shown enlarged in [B-D]). Further analysis of the rat grafts 10 weeks post-transplantation showed that many eGFP+ cells were Ki67+ as well as SSEA-1+ ([E-I]; [F-H] show enlargements of [E], and [I] is an orthogonal view; co-expression of Ki67 and eGFP appears yellow). Staining for neural progenitors showed that the grafts contained many nestin+ cells (K, L) but that few of these cells were eGFP+ (J, L). Scale bars = 100 μm (A, E) and 50 μm (D, H, L) (scale bar in [D] applies to [B, C], that in [H] applies to [F, G], and that in [L] applies to [J, K]). Abbreviations: GFP, green fluorescent protein; SSEA, stage-specific embryonic antigen.
Purification of Neurons from In Vitro Differentiated TH-eGFP Cells by Negative Selection for SSEA-1
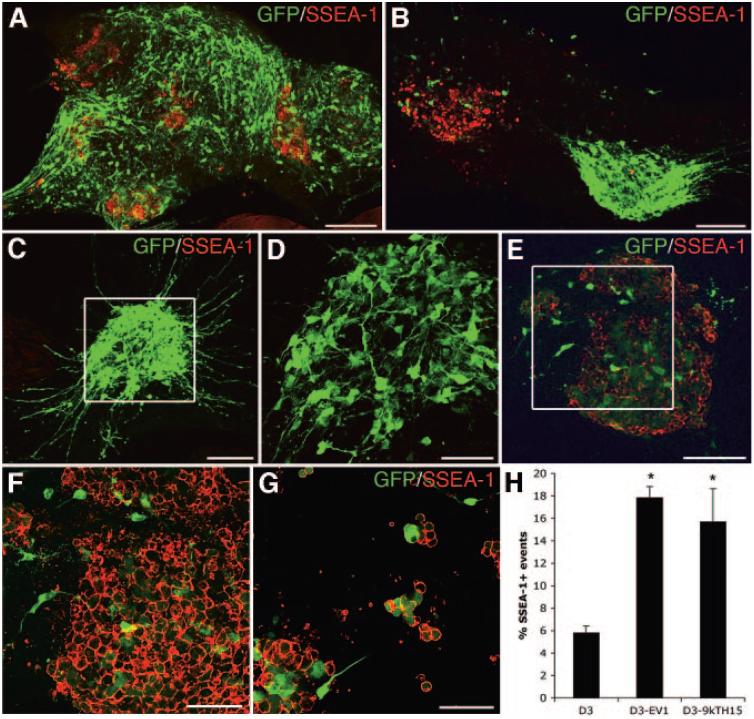
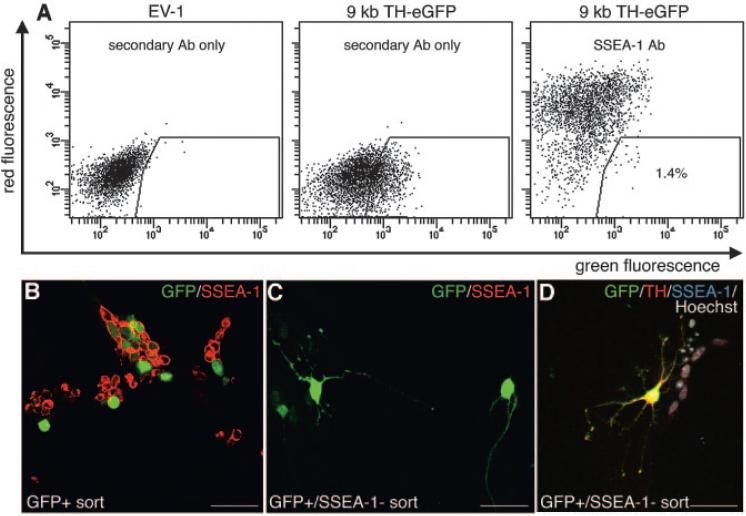
Immunofluorescent analysis of differentiated TH-eGFP mES cells showed that there were SSEA-1+ cells present in the cultures. Most individual colonies consisted of many eGFP+ cells of neuronal morphology with few or no SSEA-1+ cells. In such colonies, few or no cells were eGFP+/SSEA-1+ (Fig. 6A-6D). Some colonies, however, contained a majority of SSEA-1+ cells (Fig. 6B, 6E, 6F), and in some instances, these cells were eGFP+ (Figs 6E, 6F). These eGFP+/SSEA-1+ cells had a non-neuronal morphology and a lower expression of eGFP than the eGFP+ cells with neuronal morphology (Fig. 6E, 6F). Some of these eGFP+/SSEA-1+ cells also stained for Oct-4 (supplemental online Fig. 2). The expression of SSEA-1 was not correlated with the size of the colony, since adjacent colonies of the same size were sometimes entirely SSEA-1+ or contained only eGFP+ cells of neuronal morphology (Fig. 6B). Dissociation and replating of the TH-eGFP cultures after 9 days of differentiation, as if the cells were to be processed for FACS, surprisingly showed that most cells surviving this procedure were SSEA-1+ cells of a non-neuronal morphology (Fig. 6G). This experiment also showed that some SSEA-1+ cells expressed eGFP at a high intensity after dissociation (Fig. 6G). Live staining and FACS of differentiated TH-eGFP cells for SSEA-1 showed that the original D3 mES cell clone (n = 3) used for the genetic manipulations contained fewer SSEA-1+ events (cells) after differentiation, compared with both the empty vector (EV-1) (n = 3) and 9-kb TH-eGFP clone (n = 3) that were derived from this mES cell line (Fig. 6H). Karyotypic analysis did not reveal any acquired chromosomal abnormalities in our 9-kb TH-eGFP clone compared with the D3 mES cells (supplemental online Fig. 3), which could have explained the increase in SSEA-1+ events. To remove cells with proliferative capacity and acquire eGFP+ neurons only from differentiated TH-eGFP cells, we FACS sorted eGFP+/SSEA-1- cells. A very small fraction of cells that were present after the trypsin dissociation and the FACS procedure were eGFP+/SSEA-1- (Fig. 7A). The majority of cells sorted for eGFP alone were SSEA1+ and had a non-neuronal morphology (Fig. 7B). However, a double sort for the fraction of cells that were eGFP+/SSEA-1- (1.4% of the parental population, n = 6) resulted in a population of cells with mostly neuronal morphology (Fig. 7C, 7D). Approximately 60% of these eGFP+ neurons stained for TH (Fig. 7D).
Figure 6.
In vitro analysis of SSEA-1 expression in differentiated mouse embryonic stem (mES) cell cultures. (A-H): Analysis of in vitro-differentiated 9-kilobase (kb) TH-eGFP cells showed that most colonies contained many eGFP+ neurons and few SSEA-1+ cells and no or very few cells that were eGFP+/SSEA-1+ ([A-E]; [D] is an enlargement of [C]). Some colonies, however, contained a majority of SSEA-1+ cells ([B, E, F]; [F] is an enlargement of [E]), and in some instances, these cells were SSEA-1+/eGFP+ (E, F). The eGFP+/SSEA-1+ cells appeared to have a lower expression of eGFP than the eGFP+ cells with neuronal morphology before dissociation (C, F). The expression of SSEA-1 did not appear to be correlated with the size of the colony (B). Dissociation of cultures followed by replating showed that most cell surviving this procedure were SSEA-1+ (G). Fluorescence-activated cell sorting analysis showed that 9-kb TH-eGFP cells, as well as the EV1-transfected mES cells, contained a higher number of SSEA-1+ cells than the original D3 mES cells used for the genetic manipulations (H). Scale bars = 100 μm (A-C, E) and 50 μm (D, F, G). Abbreviations: k, kilobase; EV1, empty vector; GFP, green fluorescent protein; SSEA, stage-specific embryonic antigen; TH, tyrosine hydroxylase.
Figure 7.
Purification of neurons from in vitro-differentiated TH-eGFP cultures by negative selection for SSEA-1. Live stain and fluorescence-activated cell sorting (FACS) for eGFP+ and SSEA-1, after in vitro differentiation on MS5 for 9 days, showed that most eGFP+ cells were SSEA1+, although a small population of cells that were eGFP+/SSEA-1- could be isolated (A). The EV-1-transfected mouse embryonic stem cell clone, differentiated for the same length of time as the 9-kb TH-eGFP clone, was used as a negative control for fluorescence (A). Plating of cells sorted only for eGFP fluorescence resulted in a population consisting mainly of SSEA-1+ cells of a non-neuronal morphology (2 days in vitro post-FACS) (B). Cells negatively sorted for SSEA-1 and positively for eGFP gave a population of high neuronal purity (A, C, D). Some of the eGFP+ neurons from this double sort also expressed TH (2 days in vitro post-FACS) (D). Abbreviations: Ab, antibody; eGFP, enhanced green fluorescent protein; EV-1, empty vector; GFP, green fluorescent protein; kb, kilobase; SSEA, stage-specific embryonic antigen; TH, tyrosine hydroxylase.
Discussion
Genetic engineering of mES cells using transcription factors such as Nurr1 [2, 33], Lmx1a [34], or Pitx3 [35] can increase the number and/or the specificity of dopamine neurons generated. Addition of the BMP antagonist Noggin [36], as well as coculture with immortalized midbrain astrocytes [37], can increase the yield of dopamine neurons from human ES cells. However, ES cell cultures always contain multiple cell types after differentiation, and transplantation of such a mixed population of cells could result in tumor or teratoma formation after transplantation [5, 7, 36, 37]. In the present study, we aimed to purify mES cell-derived dopamine neurons for FACS and transplantation. We used either 2.5 or 9 kb of the rat TH promoter to drive eGFP expression and cell culture conditions favoring the generation of midbrain dopamine neurons. We found that 9-kb TH-eGFP mES clones generated high numbers of eGFP+/TH+ neurons after in vitro differentiation, sufficient for FACS, whereas 2.5-kb TH-eGFP clones did not. Based on a previous founder analysis study reporting midbrain specific reporter gene expression from the 2.5-kb TH promoter [16], we had hypothesized that this construct would label only midbrain dopamine neurons in mES cell cultures. However, in accordance with two other transgenic analyses [17, 21], this shorter promoter construct was insufficient to drive eGFP expression in an adequate way in our system. The 9-kb fragment of the rat TH promoter could, however, efficiently drive eGFP expression in dopamine neurons in mES cells, consistent with many previous transgenic analyses [11, 17, 21, 22, 38, 39]. The presence of a mix of GFP+/TH+, GFP+/TH-, and GFP-/TH+ cells in our cultures and transplants is in accordance with previous transgenic analyses using similar-size promoter fragments [11, 19, 38]. This mismatch in eGFP and TH expression could partly be explained by the insufficiency of the 9-kb promoter fragment to drive TH expression in midbrain dopamine neurons at all developmental stages. It has been shown that 9 kb of 5′ upstream sequence is adequate for the initial TH gene activation in midbrain dopamine neurons during embryogenesis and reactivation during postnatal development but insufficient for gene activation in between these time points (i.e., during the later embryonic and earlier postnatal stages) [38]. This dynamic expression of TH could in part explain the presence of TH+/eGFP- cells. Furthermore, the first intron of both the human [40-42] and the rodent [43] TH genes contain regulatory elements that appear necessary for accurate gene expression. Exclusion of intronic sequences in our promoter construct could also explain the mismatch in TH and eGFP expression [43]. The 3′ untranslated region of the TH gene is important for TH mRNA stability [44]. The lack of inclusion of 3′ untranslated sequences in our promoter construct could also partly explain the presence of TH+/eGFP- cells. In addition, eGFP reportedly has a longer half-life (>24 hours) compared with TH (16.8 hours in PC12 cells) [45], which could partly explain the presence of eGFP+/TH- neurons of a dopamine cell morphology. Once the promoter region is without activation, TH expression will fade away faster than the eGFP expression.
Transplantation of 9-kb TH-eGFP-sorted cells generated grafts with large numbers of TH neurons with an appropriate midbrain phenotype. However, our grafts contained up to 135-fold more TH neurons than expected, based on previous analyses of FACS-sorted primary embryonic TH neurons [11, 12], indicating that most cells must have been generated in vivo. The direct correlation between the number of TH neurons within the grafts and the size of the grafts supports this conclusion. This does not exclude the possibility that some of the eGFP+ neurons within the grafts originated from purified TH neurons that survived the FACS procedure and transplantation. Indeed, primary dopamine neurons can be viably isolated by FACS [9-12], although the efficiency is low. Our in vitro data suggest that mES cell-derived dopamine neurons can also survive after FACS. Additional evidence for the feasibility of viable dissociation and purification of mature, postmitotic neurons by FACS was provided in recent studies where corticospinal motor neurons from P3-P6 pups [46, 47] and striatal projection neurons from 2-month-old pups [48] were isolated. It is also evident that primary dopamine neurons can survive dissociation after in vitro culture prior to transplantation, within a certain time window [49]. This shows that even neurons that have extended long processes in vivo and/or in vitro can survive dissociation.
Despite the large number of TH neurons in the grafts, some of which extended fibers into the host, there was no general significant behavioral improvement. There were TH neurons in the grafts that extended neurites into the host, although most TH neurons did not. Improvement in amphetamine-induced rotational behavior can, however, be seen without morphological integration of grafted cells [50]. The reason we do not see improvement is likely due to the size and disruptive nature of the grafts. The lack of correlation between the total number of TH neurons in the grafts and improvement in the number of amphetamine-induced rotations supports this hypothesis. In addition, the presence of other cell types in the grafts makes it very difficult to interpret the behavior.
eGFP+ cells to be used for transplantation were sorted using a high purity mask to avoid the inclusion of eGFP- cells in our positive fraction. Reanalysis of sorted cells did, however, reveal the presence of GFP- cells in the eGFP+ sorted fraction (90% of purified cells expressed eGFP; data not shown), perhaps partly due to bleaching or cell damage. The extent of eGFP- cells in the eGFP+ fraction was similar to a previous study where teratoma formation after transplantation of 200,000 cells was avoided [8]. We cannot exclude the possibility that eGFP- cells have in part contributed to other cell types in the grafts. However, the vast majority of cells transplanted were eGFP+, and the presence of eGFP+ cells with an immature morphology co-expressing proliferative markers shows that eGFP was not only expressed in a context of mature dopamine neurons in our system. Transgenic approaches using different lengths of TH promoter sequences documented TH expression in noncatecholaminergic cells. This was originally solely explained as ectopic expression of TH due to limitations in the transgenic approaches used [11, 13, 19, 39]. However, a recent knock-in approach, where the TH gene was left undisturbed and Cre recombinase was expressed from the 3′-untransplanted region, confirmed the transient expression of TH in many noncatecholaminergic cell groups during development [51]. This transgenic analysis is also supported by previous literature documenting transient expression of TH in many cell types during embryogenesis and/or early postnatal development, where it is not found in the adult animal. Examples of peripheral tissues where TH is expressed transiently are dorsal root ganglia [52], trigeminal sensory ganglion, spiral ganglion, superior ganglion of the glossopharyngeal nerve and cells in the ventral spinal cord [51, 53], subpopulations of amacrine and interplexiform cells [54], retinal ganglion cells [51, 55], and cells lining the follicles of vibrissae [51]. TH is also transiently expressed in cells of the enteric nervous system [56-58]; precursors of insulin and glucagons cells in pancreatic islets [59]; and epithelial cells close to emerging teeth and between the fused eyelids, larynx and tongue, salivary glands, sweat glands, epididymis, and primordial follicles [51]. In the brain, TH is expressed transiently in neocortex [60], striatal cells [61], the inferior and superior colliculus [62]. In addition, TH is expressed in replicating cells of the peripheral nervous system during development [63]. Although our culture system uses Shh and Fgf8 to drive cells toward a midbrain dopamine neuron fate, other cell types are present [3]. ES cell cultures are never completely synchronized, and cells of many developmental stages are therefore present simultaneously. Although the integration site of our construct could influence the expression of eGFP, the transient expression of TH in multiple cell types during development could also explain the presence of eGFP+ cell with proliferative potential. Any approach using TH to label dopamine neurons from ES cells, whether different lengths of the promoter are used or a knock-in strategy, will generate a mixed population of cells unless the ES culture systems can be completely synchronized in time and designed to specifically generate only one cell type.
Normal transient expression of TH during development could result in the generation of eGFP+ cells of multiple cell lineages, as previously discussed. However, the expression of eGFP in a context of SSEA-1 in our cells could be due to misexpression related to transformation of the mES cells during clonal expansion. SSEA-1, a cell surface carbohydrate antigen (CD15 or Lewisx antigen), is typically expressed in preimplantation mouse embryos beginning at the eight-cell stage, in teratocarcinoma stem cells, ES cells, and adult CNS stem cells, but not in their differentiated derivatives [64-68]. Since there was no co-expression of nestin in eGFP+ cells, it is unlikely that these eGFP+/SSEA-1+ cells of immature morphology are neural precursors. Instead, the presence of higher numbers of SSEA-1+ events in the genetically engineered and expanded clones than in the original mES cell line after differentiation indicated that transformation could have taken place. Chromosomal abnormalities occur frequently in ES cells [69, 70]. Trisomy eight in mES cells is a commonly induced abnormality and is associated with an increased growth rate [69]. We therefore selected only small colonies during our initial screen. Karyotypic analysis showed that our clones had a trisomy 11 that unexpectedly was present also in the original D3 ES cells (which were only passaged four times after purchase). Therefore, this abnormality cannot explain the decreased responsiveness of our engineered clones to the dopamine fate-inducing feeders and signaling molecules. Nevertheless, these clones appear to consist of multiple populations, some of which are responsive to dopamine differentiating signals and some of which are not. These nonresponsive SSEA-1+ cells could be transformed and would subsequently lack responsiveness to any morphogen. Previous analysis of the 9-kb rat TH promoter in mES cells showed the presence of rounded eGFP+/TH- cells at the end of in vitro differentiation [71]. In that study, no further characterization of these eGFP+/TH- cells was reported, but it seems possible that some of these cells could have been SSEA-1+. In our study, we further purified the population of GFP+/SSEA-1- cells by FACS. This population had high neuronal purity and contained many cells with co-expression of eGFP and TH. The efficiency of FACS for these neuronal eGFP+ cells (SSEA-1-) was, however, too low to enable a meaningful transplantation study for in vivo analysis.
In conclusion, our study illustrates the complexity of using a single marker for selection of a specific cell population in ES cell cultures, which are not synchronized in time or specific enough to generate only one cell type. Positive selection for eGFP combined with a negative selection of an immature marker could provide an enriched neuronal population for transplantation in our context. Similar combinatorial strategies might be necessary also when other cellular markers for selection of a mature specific cell type are used.
Supplementary Material
Acknowledgments
This work was supported by Udall Parkinson's Disease Center of Excellence grant P50 NS39793 and the Orchard, Anti-Aging, and Stern foundations. E.H. was supported by a fellowship from the Swedish Brain Foundation.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST The authors indicate no potential conflicts of interest.
References
- 1.Bjorklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Auerbach JM, Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 3.Barberi T, Klivenyi P, Calingasan NY, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Hur T, Idelson M, Khaner H, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. STEM CELLS. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 5.Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 6.Thinyane K, Baier PC, Schindehutte J, et al. Fate of pre-differentiated mouse embryonic stem cells transplanted in unilaterally 6-hydroxydopamine lesioned rats: Histological characterization of the grafted cells. Brain Res. 2005;1045:80–87. doi: 10.1016/j.brainres.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda H, Takahashi J, Watanabe K, et al. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. STEM CELLS. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- 8.Chung S, Shin BS, Hedlund E, et al. Genetic selection of sox1GFPexpressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J Neurochem. 2006;97:1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.di Porzio U, Rougon G, Novotny EA, et al. Dopaminergic neurons from embryonic mouse mesencephalon are enriched in culture through immunoreaction with monoclonal antibody to neural specific protein 4 and flow cytometry. Proc Natl Acad Sci U S A. 1987;84:7334–7338. doi: 10.1073/pnas.84.20.7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr CW, Lee LJ, Romero AA, et al. Purification of dopamine neurons by flow cytometry. Brain Res. 1994;665:300–306. doi: 10.1016/0006-8993(94)91351-x. [DOI] [PubMed] [Google Scholar]
- 11.Sawamoto K, Nakao N, Kobayashi K, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson AE, Marshall CE, Yang M, et al. Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture. Mol Cell Neurosci. 2005;30:108–117. doi: 10.1016/j.mcn.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schimmel JJ, Crews L, Roffler-Tarlov S, et al. 4.5 kb of the rat tyrosine hydroxylase 5′ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Brain Res Mol Brain Res. 1999;74:1–14. doi: 10.1016/s0169-328x(99)00234-x. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee SA, Hoppe P, Brilliant M, et al. 5′ flanking sequences of the rat tyrosine hydroxylase gene target accurate tissue-specific, developmental, and transsynaptic expression in transgenic mice. J Neurosci. 1992;12:4460–4467. doi: 10.1523/JNEUROSCI.12-11-04460.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trocmé C, Sarkis C, Hermel JM, et al. CRE and TRE sequences of the rat tyrosine hydroxylase promoter are required for TH basal expression in adult mice but not in the embryo. Eur J Neurosci. 1998;10:508–521. doi: 10.1046/j.1460-9568.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Merlie JP, Todd RD, et al. Identification of cell type-specific promoter elements associated with the rat tyrosine hydroxylase gene using transgenic founder analysis. Brain Res Mol Brain Res. 1997;50:33–42. doi: 10.1016/s0169-328x(97)00163-0. [DOI] [PubMed] [Google Scholar]
- 17.Min N, Joh TH, Kim KS, et al. 5′ upstream DNA sequence of the rat tyrosine hydroxylase gene directs high-level and tissue-specific expression to catecholaminergic neurons in the central nervous system of transgenic mice. Brain Res Mol Brain Res. 1994;27:281–289. doi: 10.1016/0169-328x(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Lee JW, Chun HS, et al. Monitoring catecholamine differentiation in the embryonic brain and peripheral neurons using E. coli lacZ as a reporter gene. Mol Cells. 1997;7:394–398. [PubMed] [Google Scholar]
- 19.Kessler MA, Yang M, Gollomp KL, et al. The human tyrosine hydroxylase gene promoter. Brain Res Mol Brain Res. 2003;112:8–23. doi: 10.1016/s0169-328x(02)00694-0. [DOI] [PubMed] [Google Scholar]
- 20.Nagatsu I, Karasawa N, Yamada K, et al. Expression of human tyrosine hydroxylase-chloramphenicol acetyltransferase (CAT) fusion gene in the brains of transgenic mice as examined by CAT immunocytochemistry. J Neural Transm Gen Sect. 1994;96:85–104. doi: 10.1007/BF01277931. [DOI] [PubMed] [Google Scholar]
- 21.Sasaoka T, Kobayashi K, Nagatsu I, et al. Analysis of the human tyrosine hydroxylase promoter-chloramphenicol acetyltransferase chimeric gene expression in transgenic mice. Brain Res Mol Brain Res. 1992;16:274–286. doi: 10.1016/0169-328x(92)90236-5. [DOI] [PubMed] [Google Scholar]
- 22.Morgan WW, Walter CA, Windle JJ, et al. 3.6 kb of the 5′ flanking DNA activates the mouse tyrosine hydroxylase gene promoter without catecholaminergic-specific expression. J Neurochem. 1996;66:20–25. doi: 10.1046/j.1471-4159.1996.66010020.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim KS, Lee MK, Carroll J, et al. Both the basal and inducible transcription of the tyrosine hydroxylase gene are dependent upon a cAMP response element. J Biol Chem. 1993;268:15689–15695. [PubMed] [Google Scholar]
- 24.Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 25.Ye W, Shimamura K, Rubenstein JL, et al. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo-Sánchez M, Millet S, Simeone A, et al. Comparative analysis of Otx2, Gbx2, Pax2, Fgf8 and Wnt1 gene expressions during the formation of the chick midbrain/hindbrain domain. Mech Dev. 1999;81:175–178. doi: 10.1016/s0925-4773(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 27.Ye W, Bouchard M, Stone D, et al. Distinct regulators control the expression of the mid-hindbrain organizer signal FGF8. Nat Neurosci. 2001;4:1175–1181. doi: 10.1038/nn761. [DOI] [PubMed] [Google Scholar]
- 28.Schein JC, Hunter DD, Roffler-Tarlov S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol. 1998;204:432–450. doi: 10.1006/dbio.1998.9076. [DOI] [PubMed] [Google Scholar]
- 29.Inanobe A, Yoshimoto Y, Horio Y, et al. Characterization of G-proteingated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung CY, Seo H, Sonntag KC, et al. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: Expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- 32.Smidt MP, van Schaick HS, Lanctot C, et al. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci U S A. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung S, Sonntag KC, Andersson T, et al. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson E, Tryggvason U, Deng Q, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Chung S, Hedlund E, Hwang M, et al. The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci. 2005;28:241–252. doi: 10.1016/j.mcn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Sonntag KC, Pruszak J, Yoshizaki T, et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. STEM CELLS. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy NS, Cleren C, Singh SK, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 38.Matsushita N, Okada H, Yasoshima Y, et al. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem. 2002;82:295–304. doi: 10.1046/j.1471-4159.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- 39.Gelman DM, Noain D, Avale ME, et al. Transgenic mice engineered to target Cre/loxP-mediated DNA recombination into catecholaminergic neurons. Genesis. 2003;36:196–202. doi: 10.1002/gene.10217. [DOI] [PubMed] [Google Scholar]
- 40.Meloni R, Albanese V, Ravassard P, et al. A tetranucleotide polymorphic microsatellite, located in the first intron of the tyrosine hydroxylase gene, acts as a transcription regulatory element in vitro. Hum Mol Genet. 1998;7:423–428. doi: 10.1093/hmg/7.3.423. [DOI] [PubMed] [Google Scholar]
- 41.Albanèse V, Biguet NF, Kiefer H, et al. Quantitative effects on gene silencing by allelic variation at a tetranucleotide microsatellite. Hum Mol Genet. 2001;10:1785–1792. doi: 10.1093/hmg/10.17.1785. [DOI] [PubMed] [Google Scholar]
- 42.Lenartowski R, Goc A. Tissue-specific association of the human tyrosine hydroxylase gene with the nuclear matrix. Neurosci Lett. 2002;330:151–154. doi: 10.1016/s0304-3940(02)00746-2. [DOI] [PubMed] [Google Scholar]
- 43.Kelly BB, Hedlund E, Kim C, et al. A tyrosine hydroxylase-yellow fluorescent protein knock-in reporter system labeling dopaminergic neurons reveals potential regulatory role for the first intron of the rodent tyrosine hydroxylase gene. Neuroscience. 2006;142:343–354. doi: 10.1016/j.neuroscience.2006.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase mRNA stability by protein-binding, pyrimidine-rich sequence in the 3′-untranslated region. J Biol Chem. 1999;274:2532–2538. doi: 10.1074/jbc.274.4.2532. [DOI] [PubMed] [Google Scholar]
- 45.Wu DK, Cepko CL. The stability of endogenous tyrosine hydroxylase protein in PC-12 cells differs from that expressed in mouse fibroblasts by gene transfer. J Neurochem. 1994;62:863–872. doi: 10.1046/j.1471-4159.1994.62030863.x. [DOI] [PubMed] [Google Scholar]
- 46.Arlotta P, Molyneaux BJ, Chen J, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 47.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 48.Lobo MK, Karsten SL, Gray M, et al. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 49.Timmer M, Grosskreutz J, Schlesinger F, et al. Dopaminergic properties and function after grafting of attached neural precursor cultures. Neurobiol Dis. 2006;21:587–606. doi: 10.1016/j.nbd.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Baier PC, Schindehutte J, Thinyane K, et al. Behavioral changes in unilaterally 6-hydroxy-dopamine lesioned rats after transplantation of differentiated mouse embryonic stem cells without morphological integration. STEM CELLS. 2004;22:396–404. doi: 10.1634/stemcells.22-3-396. [DOI] [PubMed] [Google Scholar]
- 51.Lindeberg J, Usoskin D, Bengtsson H, et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 52.Jonakait GM, Markey KA, Goldstein M, et al. Transient expression of selected catecholaminergic traits in cranial sensory and dorsal root ganglia of the embryonic rat. Dev Biol. 1984;101:51–60. doi: 10.1016/0012-1606(84)90116-7. [DOI] [PubMed] [Google Scholar]
- 53.Foster GA, Schultzberg M, Dahl D, et al. Ephemeral existence of a single catecholamine synthetic enzyme in the olfactory placode and the spinal cord of the embryonic rat. Int J Dev Neurosci. 1985;3:597–608. doi: 10.1016/0736-5748(85)90050-4. [DOI] [PubMed] [Google Scholar]
- 54.Wulle I, Schnitzer J. Distribution and morphology of tyrosine hydroxylase-immunoreactive neurons in the developing mouse retina. Brain Res Dev Brain Res. 1989;48:59–72. doi: 10.1016/0165-3806(89)90093-x. [DOI] [PubMed] [Google Scholar]
- 55.Versaux-Botteri C, Verney C, Zecevic N, et al. Early appearance of tyrosine hydroxylase immunoreactivity in the retina of human embryos. Brain Res Dev Brain Res. 1992;69:283–287. doi: 10.1016/0165-3806(92)90169-w. [DOI] [PubMed] [Google Scholar]
- 56.Teitelman G, Gershon MD, Rothman TP, et al. Proliferation and distribution of cells that transiently express a catecholaminergic phenotype during development in mice and rats. Dev Biol. 1981;86:348–355. doi: 10.1016/0012-1606(81)90192-5. [DOI] [PubMed] [Google Scholar]
- 57.Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: Relationship to the development of enteric neurons. Dev Biol. 1989;132:189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- 58.Baetge G, Pintar JE, Gershon MD. Transiently catecholaminergic (TC) cells in the bowel of the fetal rat: Precursors of noncatecholaminergic enteric neurons. Dev Biol. 1990;141:353–380. doi: 10.1016/0012-1606(90)90391-u. [DOI] [PubMed] [Google Scholar]
- 59.Teitelman G, Joh TH, Reis DJ. Transformation of catecholaminergic precursors into glucagon (A) cells in mouse embryonic pancreas. Proc Natl Acad Sci U S A. 1981;78:5225–5229. doi: 10.1073/pnas.78.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satoh J, Suzuki K. Tyrosine hydroxylase-immunoreactive neurons in the mouse cerebral cortex during the postnatal period. Brain Res Dev Brain Res. 1990;53:1–5. doi: 10.1016/0165-3806(90)90119-j. [DOI] [PubMed] [Google Scholar]
- 61.Komori K, Sakai M, Karasawa N, et al. Evidence of transient expression of tyrosine hydroxylase immunoreactivity in the mouse striatum and the effects of colchicine. Acta Histochem Cytochem. 1991;24:223–231. [Google Scholar]
- 62.Jaeger CB, Joh TH. Transient expression of tyrosine hydroxylase in some neurons of the developing inferior colliculus of the rat. Brain Res. 1983;313:128–132. doi: 10.1016/0165-3806(83)90208-0. [DOI] [PubMed] [Google Scholar]
- 63.Rothman TP, Specht LA, Gershon MD, et al. Catecholamine biosynthetic enzymes are expressed in replicating cells of the peripheral but not the central nervous system. Proc Natl Acad Sci U S A. 1980;77:6221–6225. doi: 10.1073/pnas.77.10.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Natl Acad Sci U S A. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knowles BB, Pan S, Solter D, et al. Expression of H-2, laminin and SV40 T and TASA on differentiation of transformed murine teratocarcinoma cells. Nature. 1980;288:615–618. doi: 10.1038/288615a0. [DOI] [PubMed] [Google Scholar]
- 66.Fox N, Damjanov I, Martinez-Hernandez A, et al. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- 67.Eggens I, Fenderson B, Toyokuni T, et al. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem. 1989;264:9476–9484. [PubMed] [Google Scholar]
- 68.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Wu H, Loring J, et al. Trisomy eight in ES cells is a common potential problem in gene targeting and interferes with germ line transmission. Dev Dyn. 1997;209:85–91. doi: 10.1002/(SICI)1097-0177(199705)209:1<85::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 70.Sugawara A, Goto K, Sotomaru Y, et al. Current status of chromosomal abnormalities in mouse embryonic stem cell lines used in Japan. Comp Med. 2006;56:31–34. [PubMed] [Google Scholar]
- 71.Yoshizaki T, Inaji M, Kouike H, et al. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neurosci Lett. 2004;363:33–37. doi: 10.1016/j.neulet.2004.03.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.