Figure 2.
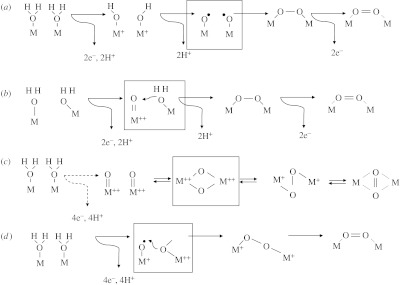
Sketches of skeletal mechanisms for formation of an O2 molecule from two molecules of H2O. Elevations in oxidation state of M are indicated by ‘+’, etc. Boxes indicate the crucial stages in each mechanism. (a,b) Ways of making an O–O bond (peroxo intermediate) that is relatively easy to oxidize further to give O2. (a) Formation of peroxo intermediate by homonuclear combination from adjacent M+−OH units deprotonated and rearranged as two oxyl radicals. (b) Formation of peroxo intermediate by attack of H2O on an electrophilic ‘O’ of [M·O]++ group. (c) Interconversion between two O2− that are either terminal ([M·O]++) or bridging ((μ-O) between two M++), an bridged between two M+, and O2 bound (weakly) between two M. (d) Attack of a bridging O2− on an oxyl radical.
