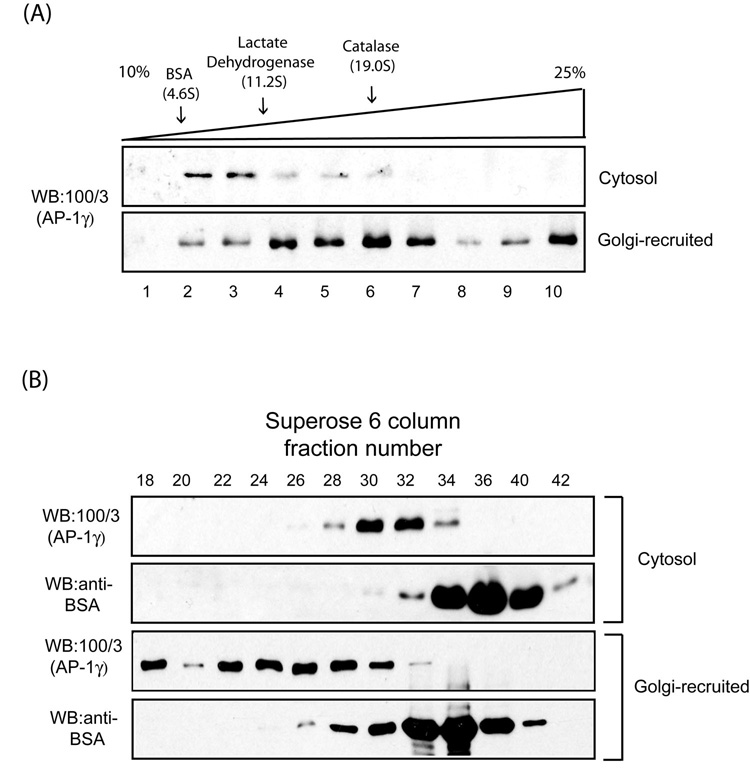
Fig. 1. AP-1 forms higher-order macromolecular complexes upon Golgi recruitment.
(A) Sucrose gradient fractionation of AP-1 recruited onto enriched Golgi membranes. 100 µg of rat liver Golgi membranes were incubated with 5 mg/ml bovine adrenal cytosol and 100 µM GTPγS for 15 min at 37 C in a 2 ml reaction. The membranes were collected by centrifugation and solubilized in assay buffer containing 2 % taurocholate. The lysate or 200 µl cytosol were loaded onto 10–25 % linear sucrose gradient and the fractions analyzed by SDS-PAGE and western blotting. The sedimentation coefficients (Svedberg units) of standard proteins run on an identical gradient are shown above. (B) Gel filtration analysis of Golgi-recruited AP-1. Golgi-recruited AP-1 was solubilized and analyzed by Superose 6 column chromatography as described in the Experimental Procedures.