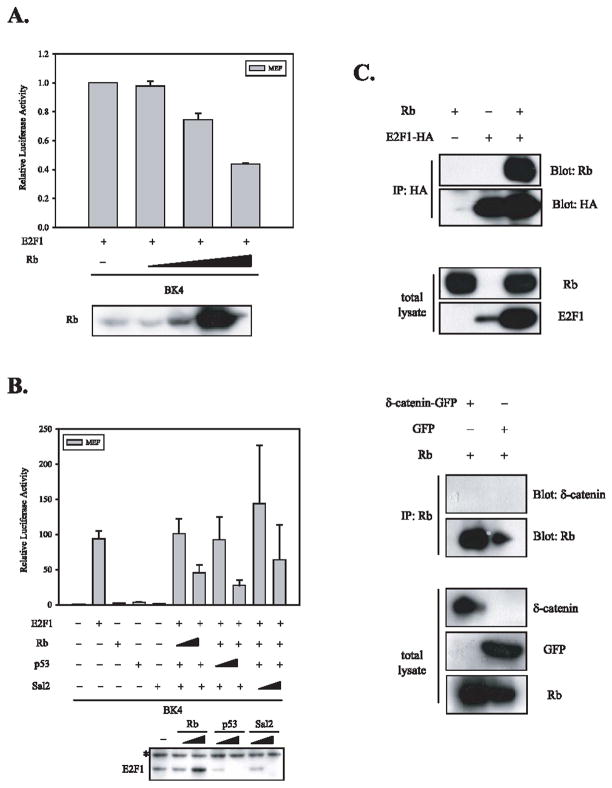
Fig. 3.
Rb suppresses the upregulation of δ-catenin-luciferase activities induced by E2F1 but does not interact with δ-catenin. (A) The increased doses of Rb suppressed the upregulation of δ-catenin-luciferase activities induced by E2F1 in a concentration dependent manner. The expression of Rb was confirmed using Western blot analysis (bottom panel). (B) Two other tumor suppressors-p53 and Sal2, implicated in the regulation of E2F, also suppress the upregulation of δ-catenin reporter activity induced by E2F1. Unlike Rb, the levels of E2F1 were dramatically reduced by increased doses of p53 and Sal2 (bottom panel). The asterisk indicates a non-specific band detected by anti-HA antibody. (C) E2F1, a well-known binding partner of Rb, was co-immunoprecipitated with Rb (top 2 panels). However, δ-catenin was not co-immunoprecipitated with Rb (bottom 2 panels). The relative expression of Rb, E2F1, δ-catenin, and GFP in total lysates was confirmed by Western blot analyses using specific antibodies against each protein.