Abstract
The PI3K-AKT pathway can mediate diverse biological responses and is crucial for optimal immune responses and lymphocyte development. Deletion of PI3K subunits or AKT leads to blockage of T cell development at the TCRβ checkpoint. Studies with over-expression of constitutively activated AKT have implicated this pathway in anti-apoptosis of developing thymocytes and in development of regulatory T cells. However, the role of endogenous PI3K-AKT in T cell development beyond the TCRβ checkpoint remains unclear. Here, we inhibited the endogenous PI3K-AKT pathway in thymocytes after DN stages by expressing the negative regulator, PTEN. These mice exhibit normal early T cell development, but the transition from ISP (Intermediate Single Positive) to DP (Double Positive) thymocytes is inhibited, leading to significant decreased number of DP, SP (Single Positive) thymocytes and peripheral T cells. Proliferation of peripheral T cells is reduced but apoptosis of DP cells and subsequent T cell maturation, including regulatory T cells, are normal. AKT phosphorylation can be readily observed in most wild-type T cell compartments but not DP thymocytes in response to TCR activation. Thus, the PI3K-AKT pathway is crucial for the transition of ISP to DP thymocytes but is dispensable for apoptosis and maturation of developing thymocytes.
Keywords: PTEN, Thymocytes, AKT, proliferation, apoptosis
Introduction
Activation of the PI3K pathway by growth factor receptors is one of the major signaling events leading to growth, proliferation and anti-apoptosis. PI3K activation results in an increase of the lipid phosphatidylinositol-3,4,5-triphosphate, which recruits lipid-binding domain containing kinases PDK1 and AKT to the membrane [1-3]. PDK1 activates AKT [4] and SGK, a serum and glucocorticoid-induced kinase with a similar structure to AKT. AKT has many direct substrates, including the cell cycle inhibitor p27, Foxo family of transcription factors, the pro-apoptotic Bcl-2 family protein Bad and TSC1, a protein that inhibits the TOR pathway [2, 4]. Phosphorylation of these substrates by AKT leads to cell cycle entry, resistance to apoptosis, increase in cell size and other growth-related events. Unlike AKT, SGK has more restricted substrates but it also phosphorylates the Foxo family of transcription factors to promote cell survival and cell cycle entry [5].
Regulation of the PI3K signaling pathway is important for normal differentiation; its dysregulation can lead to cancer. PTEN is a lipid phosphatase and a major negative regulator of the PI3K pathway [2, 6, 7]. Loss of PTEN in both alleles is frequently found in many human cancer cells. Germline heterozygous mutation of PTEN is present in patients with the Cowden syndrome [8, 9], who develop hyperplastic lesions in multiple organs with increased risks of cancer. In mice, heterozygous mutation of PTEN eventually leads to development of malignancy in different organs, including thymus, prostate, thyroid, liver and intestines [10-12]. In T cells, conditional knockout of PTEN exhibits defective apoptosis of developing thymocytes and appearance of aggressive lymphomas, resulting in mouse lethality by 15 weeks of age [13-15]. Development of tumors is preceded by activation of the PI3K pathway in the PTEN-deficient T cell precursors of these mice, including AKT phosphorylation, Foxo3a phosphorylation and reduced levels of p27 [15].
In the lymphoid system, the PI3K pathway has been shown to be important for optimal immune responses. It mediates signaling from the co-stimulatory T cell molecule CD28 and B cell docking molecules like CD19 [16-18]. During T cell development, the PI3K-AKT activities are required for the β selection checkpoint. Deletion of PDK1 or a combination of Akt1 and Akt2, two of the three AKT family members, results in a partial blockage at the DN (Double Negative CD4-CD8-) to DP (Double Positive) stage [19, 20]. Interestingly in Akt1-/-Akt2-/- thymocytes, the block at DN stage is not due to inhibition of proliferation but rather because of the loss of resistance to apoptosis when cells receive the pre-TCR signals [20]. Similarly, deletion of both PI3K catalytic subunits γ and δ results in a drastic reduction of the DP thymocyte population [21]. Although increased apoptosis of DP cells in these animals was reported [21], the defects are more likely to be similar to that of Akt1-/-Akt2-/- mice with increased apoptosis of DN population as the main culprit.
Because of the early block in DN to DP transition state in PDK1, Akt1/Akt2 or PIK3 γ/δ knockout mice, it has not been possible to properly assess the role of the PI3K-AKT pathway in later stages of T cell development. Defects detected in DP or SP (Single Positive) thymocytes in these mice could simply due to an escape of abnormal cells after the TCR β checkpoint. To overcome this problem, we generated transgenic mice expressing PTEN at DP and SP but not DN thymocytes. These mice exhibited normal number of DN thymocytes but higher number of ISP (Intermediate Single Positive) and approximately 2-fold reduction of DP and SP cells. We observed partial inhibition of TCR-stimulated proliferation of mature T cells but apoptosis of DP thymocytes was normal. AKT phosphorylation was detected in activated ISP and SP thymocytes and this was inhibited in PTEN transgenic mice. However, AKT phosphorylation was not observed in wild-type DP thymocytes, even when stimulated for 24 hours. We concluded that the PI3K-AKT pathway is important for ISP to DP transition but not for apoptosis of DP thymocytes or T cell maturation.
Results
Generation of transgenic mice over-expressing PTEN in DP, SP and mature T cells
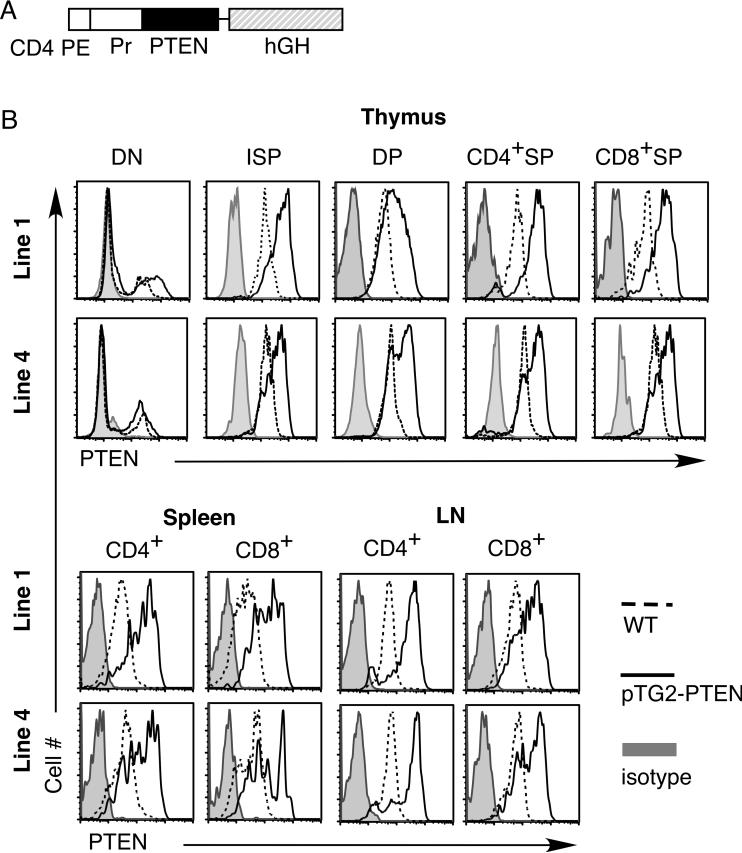
To bypass the requirement of PI3K-AKT pathway during the TCR-β checkpoint (DN3 to DN4 transition), we employed the CD4 promoter/enhancer regulatory elements [22] to drive transgenic expression of PTEN in DP, SP and mature T cells (Fig. 1A). Two transgenic founders (line 1 and line 4) were obtained and analyzed. We used intracellular staining with PTEN-specific antibody to assess expression of the PTEN transgenic protein in different T cell compartments. As expected for a transgene under the CD4 promoter/enhancer (without the CD4 silencer), no increase in PTEN expression was found in DN thymocytes but higher PTEN levels were found in ISP, DP, CD4+ and CD8+ SP transgenic thymocytes as well as mature transgenic T cells from spleen and lymph nodes (Fig. 1B).
Figure 1.
Expression of murine PTEN in different T cell populations. (A) The murine PTEN cDNA was cloned into the pTG2 vector containing the CD4 gene promoter (Pr), CD4 gene proximal enhancer (PE), and human growth hormone splicing and polyadenylation sequence (hGH). (B) Intracellular staining of PTEN expression in different T cell compartments of PTEN transgenic mice (pTG2) and their non-transgenic littermate controls (WT). Two lines of transgenic founders were shown (line 1 and line 4).
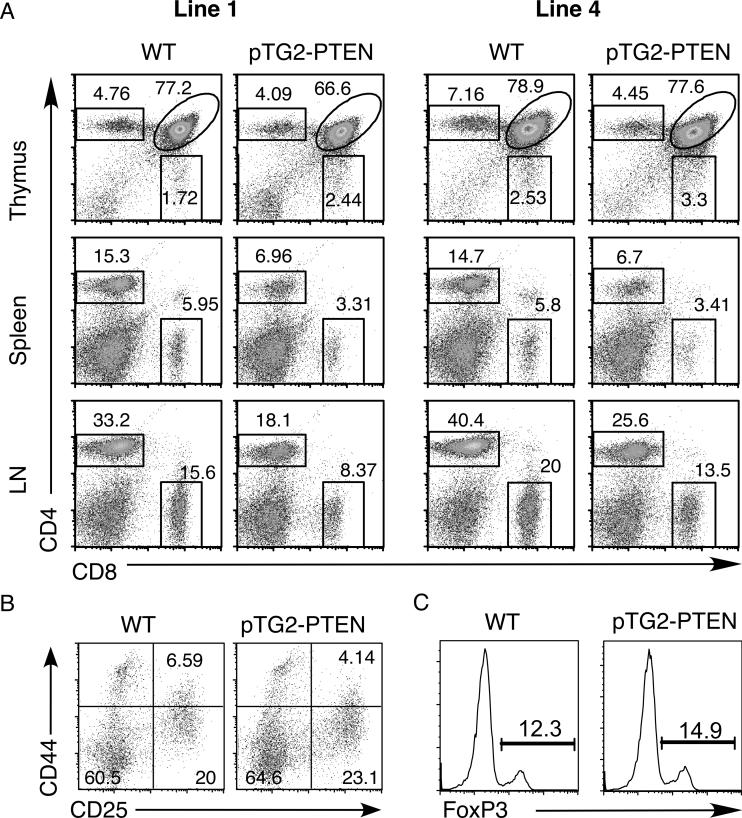
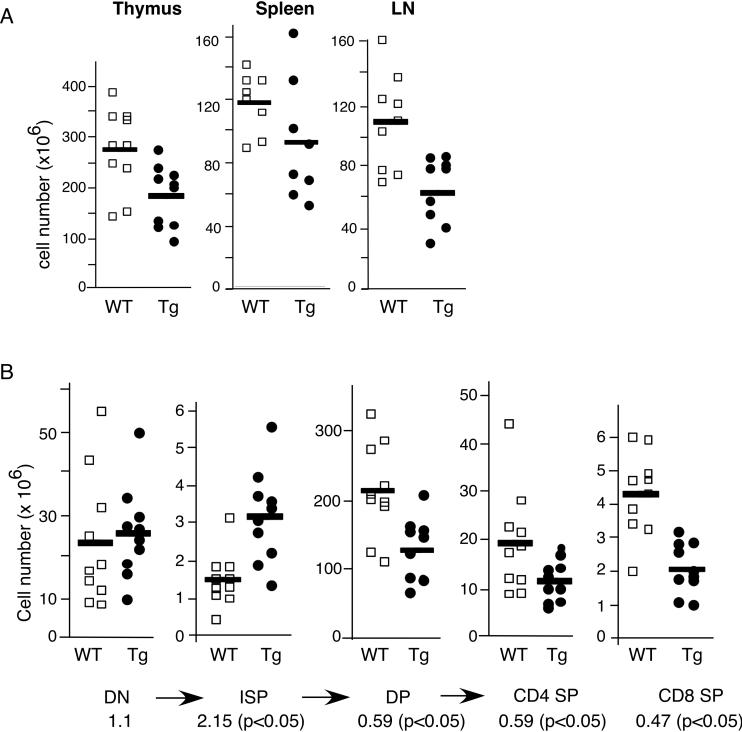
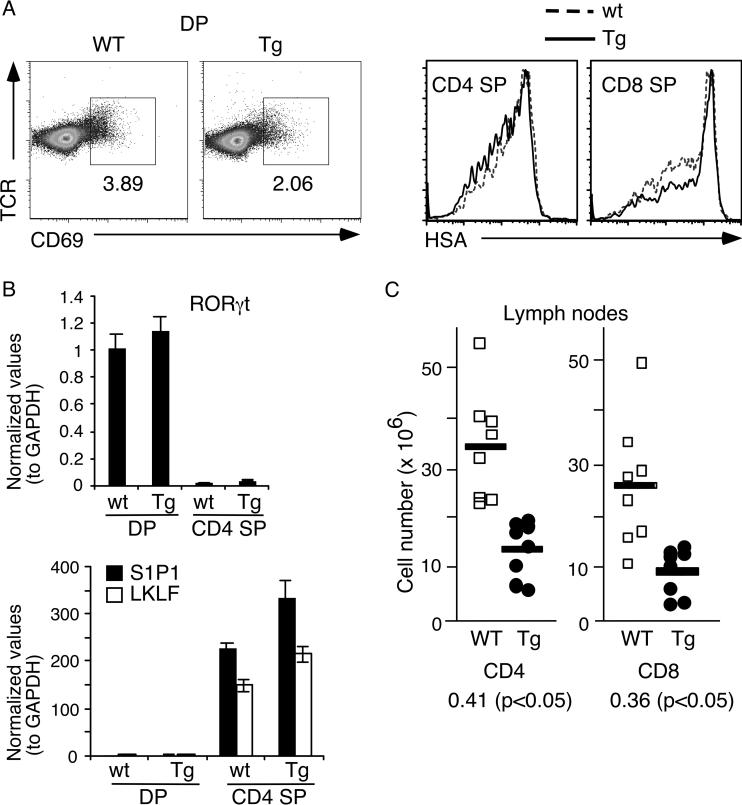
Flow cytometry analysis was carried out using antibodies specific for CD4, CD8, TCRβ and other activation markers (CD69, CD25, HSA). As shown in figure 2A, both lines of transgenic mice showed a drastic reduction of the percentages of peripheral CD4+ T cells. These mice also exhibited reduced cellularities for thymus, spleen and lymph nodes (Fig. 3A and data not shown). Adjusting for the absolute cell numbers, the PTEN transgenic mice exhibited no change in the DN thymocyte population (Fig. 3B). This was further confirmed by CD44/CD25 profile analysis of DN cells, showing normal early T cell development (Fig. 2B). However, a higher level of ISP and reduced numbers of DP, CD4+ and CD8+ SP thymocytes were observed. The increased number of transgenic ISP was approximately 2-fold (p<0.05) (Fig. 3B). As ISP thymocytes are the intermediate population between DN and DP cells [23], these data suggest that inhibition of the PI3K-AKT pathway results in a blockage of ISP to DP transition, leading to an accumulation of the ISP population and a 1.67-fold reduction of DP, CD4+ SP T cells and a 2-fold reduction in CD8+ SP cells (Fig. 3B). Positive selection is largely normal as indicated by a similar drop of the number of SP thymocytes as that of DP cells. We also performed staining with CD69 and HSA-specific antibodies. CD69 is usually up-regulated in thymocytes receiving TCR signals and HSA is down-regulated upon positive selection. Although the level of CD69 was slightly lower for the PTEN transgenic thymocytes (Fig. 4A left panels), their HSA level was indistinguishable from the control mice (Fig. 4A, right panels). In addition, the expression levels of RORγt, a transcriptional factor crucial for the survival of DP thymocytes [24], were similar between transgenic and wild-type DP thymocytes (Fig. 4B). To examine thymic emigration, we measured the amount of mRNA for S1P1 and LKLF, two genes essential for emigration of SP thymocytes to the peripheral organs [25, 26]. As shown in figure 4B, no significant differences were found in S1P1 and LKLF expression levels between PTEN transgenic and WT littermate controls. Consistent with the lack of major defects in emigration, the drop in the absolute cell numbers of transgenic CD4 and CD8 T cells in the lymph nodes and spleen were roughly comparable to the drop of the transgenic SP thymocytes (Fig. 4C and data not shown). Finally intracellular staining with Foxp3 antibody showed normal percentage of regulatory T cells in the splenic CD4+ cells of these PTEN mice (Fig. 2C).
Figure 2.
Flow cytometric profiles of pTG2-PTEN transgenic mice (Tg). (A) CD4 versus CD8 profiles of thymus, spleen and lymph nodes (LN) for line 1 and line 4 pTG2-PTEN transgenic mice as well as their littermate control mice (WT). (B) CD44 versus CD25 profiles of gated DN thymocytes. (C) Foxp3 intracellular staining of splenic CD4+ T cells. The experiments were done more than three times with similar results.
Figure 3.
The total number of cells found in the lymphoid organs from pTG2-PTEN transgenic mice (Tg) and littermate controls (WT). (A) The total number of cells found in thymus, spleen and lymph nodes (LN) of pTG2-PTEN transgenic mice and their littermate controls (WT). Each box or circle represents one mouse (n=10). (B) The number of cells in each thymocyte population from transgenic and WT littermates (n=10). CD4 and CD8 SP thymocytes were gated on TCRhigh population. The ratio of transgenic/non-transgenic cell number for each cell population is indicated below (p values were measured using student's t-test). The data was based on 10 pairs of mice. The arrows represent the order of T cell differentiation.
Figure 4.
Normal positive selection and thymic emigration in pTG2-PTEN transgenic mice (A) Left two panels: CD69 versus TCR staining profiles of DP thymocytes in transgenic (Tg) and their littermate controls (WT). Right two panels: HSA staining of CD4 SP and CD8 SP thymocytes in transgenic mice and their littermates. (B) Quantitative real-time PCR of RORγt, S1P1, and LKLF using sorted DP thymocytes and CD4 SP thymocytes. (C) The total cell number of CD4+ and CD8+ lymph node T cell population from transgenic and WT littermate controls (n=8). The ratio of transgenic/non-transgenic cell number for each cell population is indicated below the graph (p values were measured using Student's t-test). The experiments in panel A were done more than 3 times with similar results, those in panel B were based on triplicate samples and data in panel C were based on 8 pairs of mice.
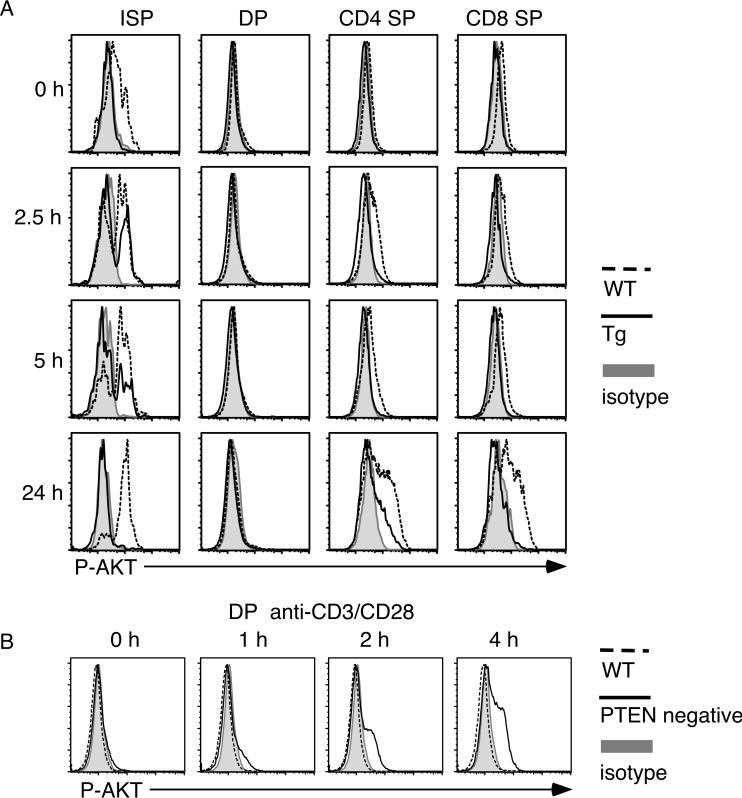
AKT phosphorylation in ISP and TCR-stimulated SP but not DP thymocytes
To examine activation of the PI3K-AKT pathway in different T cell populations and to assess the effect of PTEN over-expression, we performed intracellular staining with phospho-AKT-specific antibody. Thymocytes were stimulated with anti-CD3/CD28 for various time points and stained with CD4, CD8, TCRb and P-AKT specific antibodies. As shown in figure 5A, ISP thymocyte population (CD8+CD4-TCRβlow) from wild-type mice exhibited a small but consistent constitutive AKT phosphorylation in unstimulated population. This is consistent with the known proliferating nature of ISP [23]. Further TCR stimulation led to a small increase of AKT phosphorylation. As expected, AKT phosphorylation was greatly diminished in transgenic ISP population, indicating the efficient repression of the PI3K-AKT pathway by the transgenic PTEN protein. Repression of this pathway was also observed in TCR stimulated CD4 or CD8 SP thymocytes. After 24-hour stimulation, high levels of PAKT were seen in CD4 and CD8 SP cells from wild-type mice. Residual P-AKT levels were seen in transgenic CD4 SP cells but none was seen in transgenic CD8 SP thymocytes (Fig. 5A). In contrast, stimulated DP thymocytes didn't exhibit any AKT phosphorylation at all time points, even in wild-type cells (Fig. 5A). This result was further confirmed by western blot using stimulated sorted wild-type DP thymocytes (data not shown) and by the readily detectable AKT phosphorylation in PTEN-deficient DP thymocytes (Fig. 5B)
Figure 5.
Phosphorylation kinetics of AKT following TCR stimulation. (A) Thymocytes from PTEN transgenic (Tg) or WT littermates were stimulated with plate-bound anti-CD3/CD28 antibodies for the indicated time points. Cells were fixed and stained with antibodies specific for CD4, CD8, TCRβ and phospho-AKT. (B) Thymocytes from PTEN-deficient mice (PTENflox/flox/lck-Cre) [15] or wild-type littermates were stimulated with plate-bound anti-CD3/CD28 antibodies for the indicated time points. Cells were fixed and stained with antibodies specific for CD4, CD8 and phospho-AKT. Cells were analyzed by flow cytometry. The experiments were done more than three times with similar results.
Normal DP thymocyte apoptosis in pTG2-PTEN transgenic mice
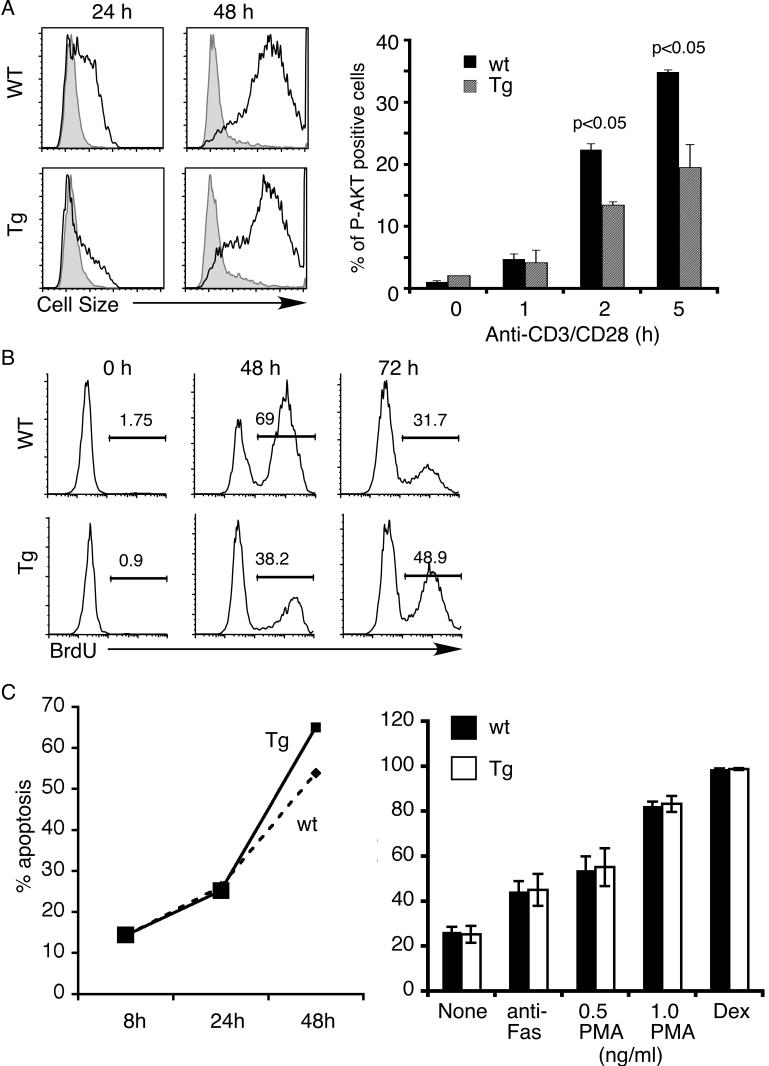
To further examine the effect of PTEN over-expression on proliferation and apoptosis, we isolated total splenocytes from transgenic mice and their wild-type littermates. Splenocytes were then stimulated with soluble anti-CD3/CD28 antibodies for up to 72 hours. Proliferation was assessed using BrdU incorporation and through increased cell size. While wild-type T cells increased their cell size by 24 hours, only a small proportion of transgenic T cells had done so (Fig. 6A). By 48 hours, however, both wild-type and transgenic T cells exhibited similar blasts. A similar delay in proliferation kinetics was seen in experiments examining BrdU incorporation (Fig. 6B). A two-fold reduction in BrdU incorporation was observed in transgenic T cells stimulated for 48 hours but the difference disappeared by 72 hours. The partial nature of the effect of PTEN was confirmed by examining AKT phosphorylation. As shown in figure 6A (right panel), a partial inhibition of AKT phosphorylation was observed in transgenic T cells. Akt phosphorylation could be detected within the first 5 hours of TCR stimulation but the extent of phosphorylation was much lower after 24-hour stimulation.
Figure 6.
Transgenic expression of PTEN affected proliferation of peripheral transgenic T cells but not apoptosis of transgenic DP thymocytes. (A & B) PTEN transgenic splenic T cells exhibited partial defects in TCR-induced proliferation. Total splenocytes from PTEN transgenic mice or their wild-type littermate controls were stimulated with soluble anti-CD3/CD28 antibodies for the indicated time points. CD4+ T cells were gated and analyzed. (A) Left panel: cell size as measured by side scatter (forward scatter showed a similar pattern). Right panel: the level of AKT phosphorylation was measured using intracellular staining with P-AKT specific antibodies. (B) Proliferation was measured using BrdU incorporation. The percentages of BrdU+ cells are as shown in the figure. (C) Normal apoptosis of PTEN transgenic DP thymocytes. Left panel: apoptosis of DP thymocytes culture in vitro over time. Right panel: DP thymocytes from PTEN transgenic mice (Tg) or their wild-type littermate controls were stimulated with anti-Fas antibody, PMA phorbol ester or dexamethasone (Dex). The extent of apoptosis (% Annexin V positive, 7-AAD negative cells) was measured 16 hours later.
Finally, we examined apoptosis of transgenic DP thymocytes in vitro with or without stimulation with anti-Fas antibody, phorbol ester PMA or dexamethasone. Apoptotic cells were scored as those that are AnnexinV positive and 7-AAD negative. No significant difference in the rate of apoptosis was found in cultured thymocytes at all the time points examined (Figure 6C, left panel). Similarly, apoptosis through the Fas pathway, by PMA or by dexamethasone proceeded normally in the transgenic DP cells (Figure 6C, right panel).
Discussion
The role of PI3 kinase in T cell function and early T cell development has been studied extensively but whether this is an obligatory pathway for T cell development beyond the β checkpoint is not clear. PI3 kinase pathway is important for co-stimulatory activity of mature T cells. Expression of activated AKT protein results in T cells that are CD28-independent [17] and exhibit resistance to Fas-induced apoptosis [27]. Deletion of the PI3 kinase subunits delta/gamma or the downstream kinases AKT1/2 leads to abnormal transition of DN to DP stage [20, 21]. We show here that over-expression of PTEN in T cells after the β checkpoint leads to a partial block at the transition of ISP to DP thymocytes and subsequently decreased of DP and SP thymocytes, but positive selection is normal. During T cell development two checkpoints, β selection and positive/negative selection, have been extensively studied. However, the molecular mechanisms of DN to ISP and ISP to DP transitions are poorly understood. Only IL-7R and two families of transcription factors, TCF-1 and RORγ have been implicated in this process [28-32]. Mice with null mutations at TCF-1 and its family member, LEF1, in particular, exhibit a complete block of ISP to DP transition [29, 30]. Our data here suggest that the PI3 kinase pathway also plays an important role during the transitional stage from ISP to DP. As AKT has been shown to inhibit GSK3, a negative inhibitor of the β-catenin/TCF-1/LEF-1 pathway [33], decreased AKT phosphorylation in PTEN transgenic mice might lead to lower levels of β-catenin/TCF-1 transcriptional activity and subsequent inhibition of ISP to DP transition similar to those seen at the TCF-1 knockout mice [29].
We also show that apoptosis of PTEN transgenic DP T cells appears to be normal. Apoptosis of PTEN transgenic DP cells is similar to non-transgenic DP cells when cultured in vitro or when stimulated with dexamethasone, phorbol ester or Fas. This is in contrast to thymocytes with deletion of the PI3 kinase subunits delta/gamma, which are more susceptible to cell death [21]. We think that the discrepancy might be due to the abnormal DN to DP transition in PI3Kγ/PI3Kδ knockout mice, which selects unusual population of DP cells that are more sensitive to cell death. Our results are consistent with the inability to detect AKT phosphorylation in DP thymocytes, even when they are stimulated for various length of time (this paper figure 5A, [34]). AKT phosphorylation could only be seen in DP cells when PTEN is missing, suggesting that PTEN plays a major role in regulating AKT responsiveness in DP cells. The inability to detect AKT phosphorylation in DP cells is somewhat surprising, given the previous suggestions that the PI3K-AKT pathway is important for apoptosis. However, the conclusion that the PI3K-AKT pathway is crucial for DP cells was derived from studies utilizing over-expression of activated AKT (and thus did not address the endogenous pathway) or from knockout thymocytes that have defects in the earlier stage of T cell development [21, 35]. In contrast to DP cells, AKT activation can be observed readily in ISP. The ISP population contains mostly proliferating cells and express high levels of cell cycle proteins c-myc [36] and Ki-67 [15]. Inhibition of ISP to DP transition by transgenic PTEN is consistent with the role of AKT in proliferation, perhaps through the β-catenin/TCF-1 pathway as discussed earlier.
Recently, the AKT-mTOR pathway was shown to affect regulatory T cell production during T cell development [37]. Introduction of activated AKT retroviruses leads to inhibition of Foxp3+ regulatory T cells. In pTG2-PTEN mice, however, we detected no changes in the number of Foxp3+ cells, thus the PI3K pathway is not required for Treg development although AKT activation can negatively affect Treg. This might work by increasing the frequency of thymocytes that undergo positive/negative selections. It is also interesting to note that loss of PTEN in DP cells results in resistance to negative selection [13] although expression of activated AKT has no consistent effect on negative selection [38]. Thus, other proteins like SGK [1, 39] might play a more major role in DP negative selection. Additional experiments will be necessary to dissect out the role of individual players in the PI3K signal transduction pathway during T cell development.
Materials and methods
Generation of PTEN transgenic mice
The pTG2 vector was constructed using the murine CD4 gene promoter, the proximal enhancer, and the human β-globin genes from p1017 [40] based on previous transgenic studies of the CD4 regulatory elements [22]. The murine CD4 promoter (983 bp) and the enhancer (339 bp) were amplified from B6 genomic DNA using the following oligonucleotides: promoter forward (5'-AGAAACTGCAGATCTGGGCTAGAGGAGAATATGG) and reverse (5'-AACGCGGATCCGTTAACCTCGAGACTTTGCAAACAGG), enhancer forward (5'-ATAAGAATGCGGCCGCTGTTGGGGTTCAAATTTGA) and reverse (5'-AGAAACTGCAGACCAATCTACCTCCACCCTGGCT). The enhancer and the promoter were ligated to each other after the enhancer was digested with NotI/PstI and the promoter was digested with PstI/BamHI. The enhancer-promoter fragment was then cloned into NotI/partial BamHI-digested p1017. Unique sites in this pTG2 transgenic constructs are SalI and BamHI. To generate PTEN transgenic mice, the murine PTEN cDNA (Open Biosystem) was amplified by PCR and subcloned into the pTG2 vector. Transgenic mice were generated using standard procedures. Transgenic mice were genotyped by PCR using the following primers: hgh 5' (GACAC AAACT CACAC AACGA TGACG C) and hgh 3' (ATGCC TGGAA CTCCA ACAAC TCGG).
Flow cytometry
Cells were prepared from lymphoid organs of littermates. After red blood cell lysis, they were stained with the indicated antibodies. Anti-CD4, anti-CD8, anti-TCRβ, anti-CD69, anti-CD25, and anti-HSA antibodies were purchased from BD Pharmingen. For the Foxp3 staining, total splenocytes were fixed and stained with anti-CD4 and anti-mouse Foxp3 (eBioscience) according to the manufacturer instructions.
Intracellular staining
Fresh isolated thymocytes and peripheral T cells were fixed and stained with anti-PTEN antibody. Thymocytes were stimulated with plate-bound anti-CD3 (2 μg/mL) and anti-CD28 (2 μg/mL) for the indicated time periods, fixed and stained with anti-phospho-Akt antibody. Total splenocytes were stimulated with soluble anti-CD3 (0.5 μg/mL) and anti-CD28 (0.5 μg/mL) for the indicated time periods, fixed and stained with anti-phospho-Akt antibody. Intracellular staining of phospoh-Akt and PTEN was performed as described previously [41]. Briefly, formaldehyde was added directly to culture medium to a final concentration of 2% and incubated for 10 min at room temperature. The cells were pelleted, resuspended in ice-cold methanol, and incubated for 15-30 min on ice. Then the cells were washed three times with staining buffer (0.5% BSA in PBS) and stained with antibodies. Anti-phospho AKT (ser 473) and anti-PTEN antibodies were purchased from Cell Signaling.
Quantitative real-time RT-PCR
Total RNAs were extracted from sorted DP thymocytes and CD4+CD8- SP thymocytes. The primer sequences are: RORγt forward (5'-TGTGCAGATCTAAGGGCTGA) and reverse (5'-CAGTAGGGTAGCCCAGGACA), S1P1 forward (5'-GTGTAGACCCAGAGTCCTGCG) and reverse (5'-AGCTTTTCCTTGGCTGGAGAG), LKLF forward (5'-CGCCACTACCGAAAGCAC) and reverse (5'-CGCACAAGTGGCACTGAAAG), as well as GAPDH forward (5'-AGAACATCATCCCTGCATCC) and reverse (5'-CACATTGGGGGTAGGAACAC).
BrdU incorporation
Total splenocytes were stimulated with soluble anti-CD3 (0.5 μg/mL) and anti-CD28 (0.5 μg/mL) for the indicated time periods and 10 μM of BrdU was added into cell culture medium for the last 16 h. The BrdU staining was performed after cells were fixed. The anti-BrdU antibody was purchased from Caltag.
Thymocyte culture and apoptosis assay
Thymocytes was isolated and stimulated with anti-Fas (2 μg/mL), PMA (0.5, 1 ng/mL) or Dexamethasone (7.8 nM). After the indicated time, cells were collected and stained with cell surface markers, CD4 and CD8, as well as Annexin V (BD Biosciences) and 7-AAD for flow cytometric analysis.
Acknowledgements
We thank M. Adlam and G. Siu for their advice in CD4 regulatory elements and the rest of the Winoto group for discussion. This work is supported by a grant from NIH (to AW).
Abbreviations
- DN
double negative CD4-CD8-
- ISP
Intermediate single positive CD8+CD4-CD3low
- DP
Double Positive CD4+CD8+
- SP
single positive (CD4+CD8- or CD4-CD8+).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 3.Juntilla MM, Koretzky GA. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol. Lett. 2008;116:104–110. doi: 10.1016/j.imlet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J. Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 6.Rossi DJ, Weissman IL. Pten, tumorigenesis, and stem cell self-renewal. Cell. 2006;125:229–231. doi: 10.1016/j.cell.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, Lindboe CF, Fryns JP, Sijmons RH, Woods DG, Mariman EC, Padberg GW, Kremer H. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum. Mol. Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- 9.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 10.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 12.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 14.Hagenbeek TJ, Spits H. T-cell lymphomas in T-cell-specific Pten-deficient mice originate in the thymus. Leukemia. 2008;22:608–619. doi: 10.1038/sj.leu.2405056. [DOI] [PubMed] [Google Scholar]
- 15.Xue L, Nolla H, Suzuki A, Mak TW, Winoto A. Normal development is an integral part of tumorigenesis in T cell-specific PTEN-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2022–2027. doi: 10.1073/pnas.0712059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckler JL, Walsh PT, Porrett PM, Choi Y, Turka LA. Cutting Edge: T Cell Requirement for CD28 Costimulation Is Due to Negative Regulation of TCR Signals by PTEN. J. Immunol. 2006;177:4262–4266. doi: 10.4049/jimmunol.177.7.4262. [DOI] [PubMed] [Google Scholar]
- 17.Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur. J. Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 18.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat. Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 19.Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nat. Immunol. 2004;5:539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- 20.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swat W, Montgrain V, Doggett TA, Douangpanya J, Puri K, Vermi W, Diacovo TG. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adlam M, Siu G. Hierarchical interactions control CD4 gene expression during thymocyte development. Immunity. 2003;18:173–184. doi: 10.1016/s1074-7613(03)00021-9. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald HR, Budd RC, Howe RC. A CD3- subset of CD4-8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur. J. Immunol. 1988;18:519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 24.Winoto A, Littman DR. Nuclear Hormone Receptors in T Lymphocytes. Cell. 2002;109:S57–S66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 25.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 26.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 27.Jones RG, Elford AR, Parsons MJ, Wu L, Krawczyk CM, Yeh WC, Hakem R, Rottapel R, Woodgett JR, Ohashi PS. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J. Exp. Med. 2002;196:335–348. doi: 10.1084/jem.20020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q, Erman B, Park JH, Feigenbaum L, Singer A. IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORgammat: impact on thymocyte development. J. Exp. Med. 2004;200:797–803. doi: 10.1084/jem.20032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 30.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRα gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Deftos ML, Ojala EW, Bevan MJ. RORγt, a Novel Isoform of an Orphan Receptor, Negatively Regulates Fas Ligand Expression and IL-2 Production in T Cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 33.Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev. Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham NR, Artim SC, Fornadel CM, Sellars MC, Edmonson SG, Scott G, Albino F, Mathur A, Punt JA. Immature CD4+CD8+ Thymocytes and Mature T Cells Regulate Nur77 Distinctly in Response to TCR Stimulation. J. Immunol. 2006;177:6660–6666. doi: 10.4049/jimmunol.177.10.6660. [DOI] [PubMed] [Google Scholar]
- 35.Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J. Exp. Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CY, Bredemeyer AL, Walker LM, Bassing CH, Sleckman BP. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. Eur. J. Immunol. 2008;38:342–349. doi: 10.1002/eji.200737972. [DOI] [PubMed] [Google Scholar]
- 37.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Na SY, Patra A, Scheuring Y, Marx A, Tolaini M, Kioussis D, Hemmings B, Hunig T, Bommhardt U. Constitutively active protein kinase B enhances Lck and Erk activities and influences thymocyte selection and activation. J. Immunol. 2003;171:1285–1296. doi: 10.4049/jimmunol.171.3.1285. [DOI] [PubMed] [Google Scholar]
- 39.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen JM, Forbush KA, Perlmutter RM. Functional dissection of the lck proximal promoter. Mol. Cell. Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krutzik PO, Clutter MR, Nolan GP. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J. Immunol. 2005;175:2357–2365. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]