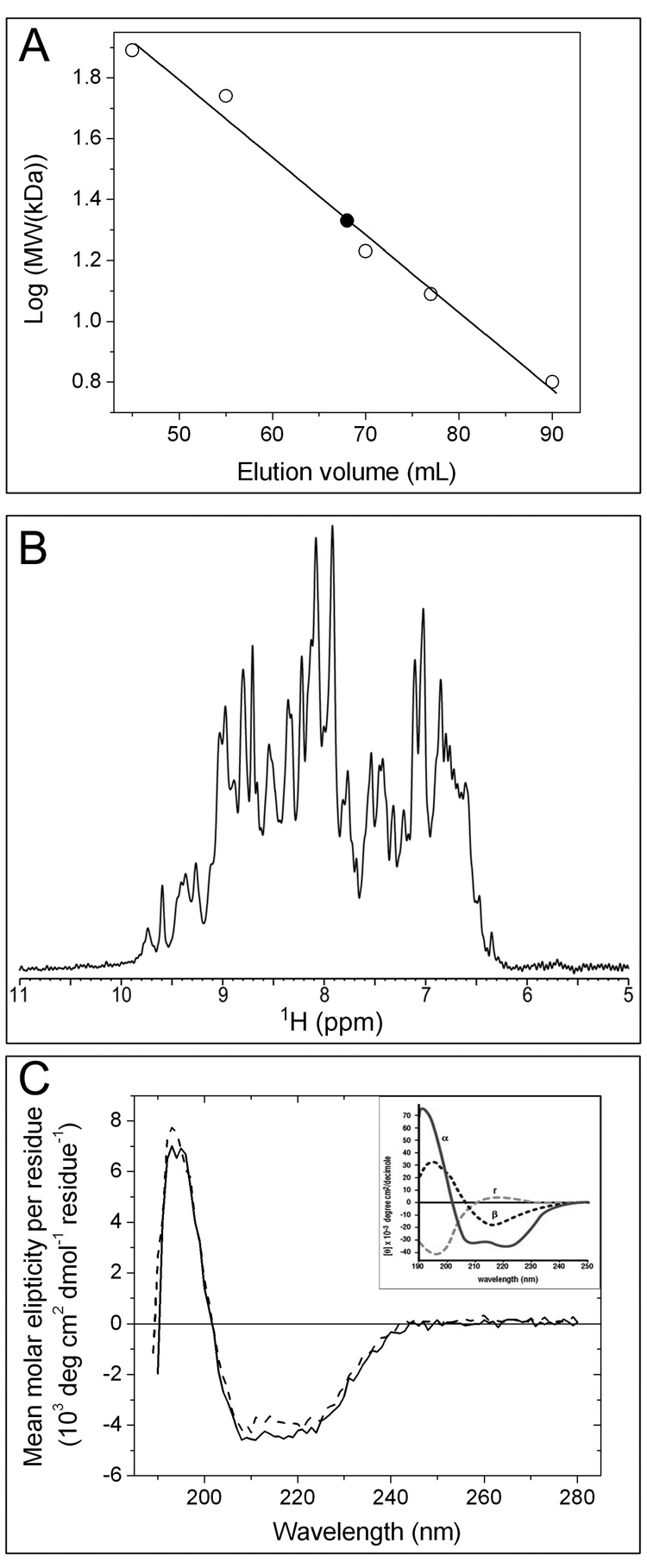
Figure 6.
Characterization of the solution properties of CzrBsf. (A) Size exclusion chromatography. Apo-CzrBsf eluted at an included volume of 68 mL (filled square) corresponding to a molecular weight of 21.6 kD. The identity and molecular weight of the standard proteins used to calibrate the column (open squares) follow: conalbumin (77 kDa), hyaluronidase (55 kDa), myoglobin (16.9 kDa), cytochrome c (12.4 kDa), and GB1 (6.2 kDa). The line of best fit to the data is shown as a solid line., (B) 1H Nuclear magnetic resonance. The amide and aromatic region of the 1H NMR spectrum of ~1 mM apo-CzrBsf at 25 °C and pH 6.5 is shown. (C) Circular dichroism spectra for secondary structure analysis of the His-tagged protein are shown before (solid line) and after lyophilization (dashed line). The inset shows for comparison the circular dichroic behavior of proteins exclusively in the α-helical (α), antiparallel β-sheet (β) and random coil (r) conformations (from ‘Protein Structure and Function’, ed. Petsko GA and Ringe D, 2004).