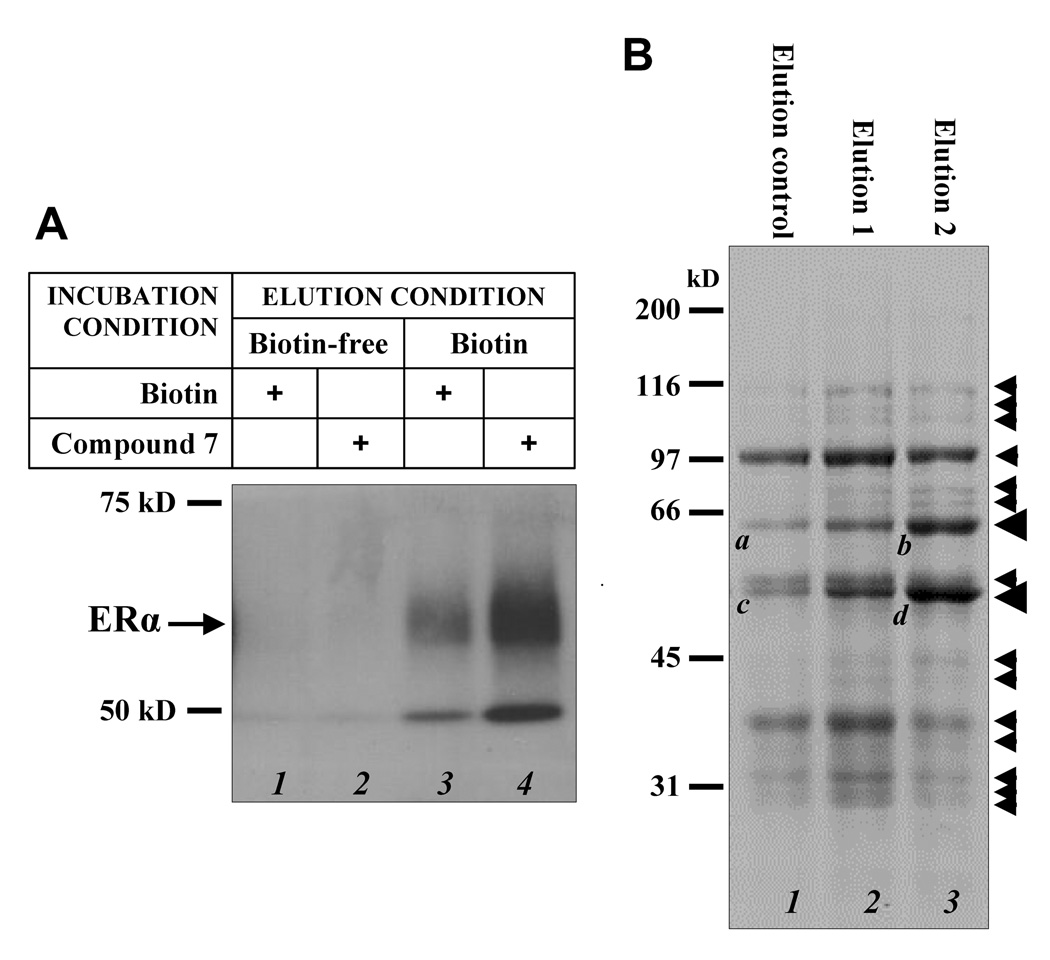
Figure 2.
Use of compound 7 for the isolation of human ERα and other E2-binding proteins from human MCF-7 cells. A. Western blot analysis of the eluted fractions from affinity chromatography of human recombinant ERα protien after incubation with either biotin alone (lanes 1 and 3) or compound 7 (lanes 2 and 4). The avidin-immobilized affinity column was first eluted with biotin-free solution (lanes 1 and 2) and then followed by 2 mM biotin-containing solution (lanes 3 and 4). The proteins in the collected fractions were separated by SDS-PAGE electrophoresis. B. SDS-PAGE analysis of potential E2-binding proteins isolated from cytosolic proteins of MCF-7 cells. After incubation of crude cytosolic protein mixtures with compound 7 or biotin, the mixture was loaded onto the affinity column and eluted sequentially with an E2-free elution buffer (elution control), 20 µM E2-containing elution buffer (elution 1), or after incubation at 4°C for 6 hrs with 20 µM E2 and then eluted with the E2-containing buffer (elution 2). The small arrows label the proteins that appear to be preferentially eluted off with an E2-containing buffer either during the first elution or during the second elution. The two quantitatively-major bands with a fat arrowhead were further analyzed with LC-MS/MS for structural compositions. The data are shown in Table 1.