FIGURE 1. Models for regulation of receptor tyrosine kinases and SFK-dependent non-catalytic receptors.
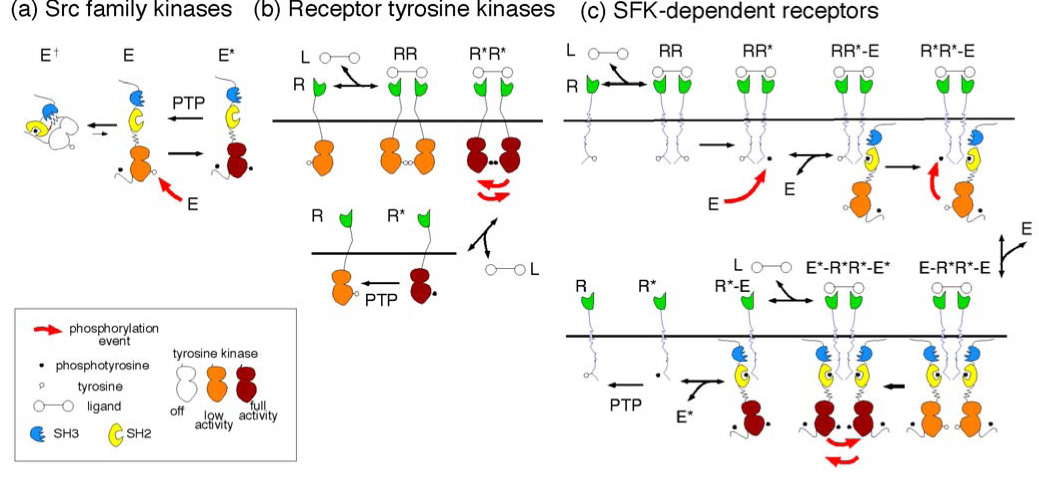
(a) Structure and regulation of SFKs. SFKs have 3 domains: SH3 (blue, phosphorylation-independent binding, not considered here), SH2 (yellow, phosphorylation-dependent binding) and kinase (white, orange or crimson according to increasing activity). The SFK can adopt two main conformation states: "closed" inactive (E†) and "open" low (E) and high (E*) activity forms. E is converted to E* by intermolecular phosphorylation in the activation loop. In addition, phosphorylation at the C terminus alters the balance between the closed and open conformations by stabilizing the closed form. This phosphorylation is not regulated in the model, so is not considered further.
(b) Regulation of RTKs. The kinase domains of monomeric RTKs, R, are inhibited by intramolecular interactions, and have low basal activity (orange) which is readily reversed by PTPs. Following dimerization by ligand, L, intermolecular phosphorylation of the activation loop occurs, and the kinase is activated (crimson). If ligand dissociates, monomeric receptors will remain in the phosphorylated, active state until dephosphorylated by PTPs. This allows for hysteresis in signaling.
(c) Proposed model for regulation of SDRs. Only a small fraction of monomeric SDRs (R) are phosphorylated. After dimerization by ligand, this phosphorylation may allow binding of an SFK (RR*-E complex). This may lead to an "intramolecular" phosphorylation to form a R*R*-E complex. This stimulates receptor phosphorylation. If a second E binds, to form an E-R*R*-E complex, then intermolecular phosphorylation of E will be stimulated, and the E*-R*R*-E* complex will have high kinase activity. Phosphorylated E* may be released into the cytosol, to phosphorylate more receptors. This would provide a mechanism for hysteresis in signaling.
