Figure 4. Protein-protein interaction of Ugene-p and UNG2 demonstrated by serial co-immunoprecipitation (Co-IP) Western blot assay.
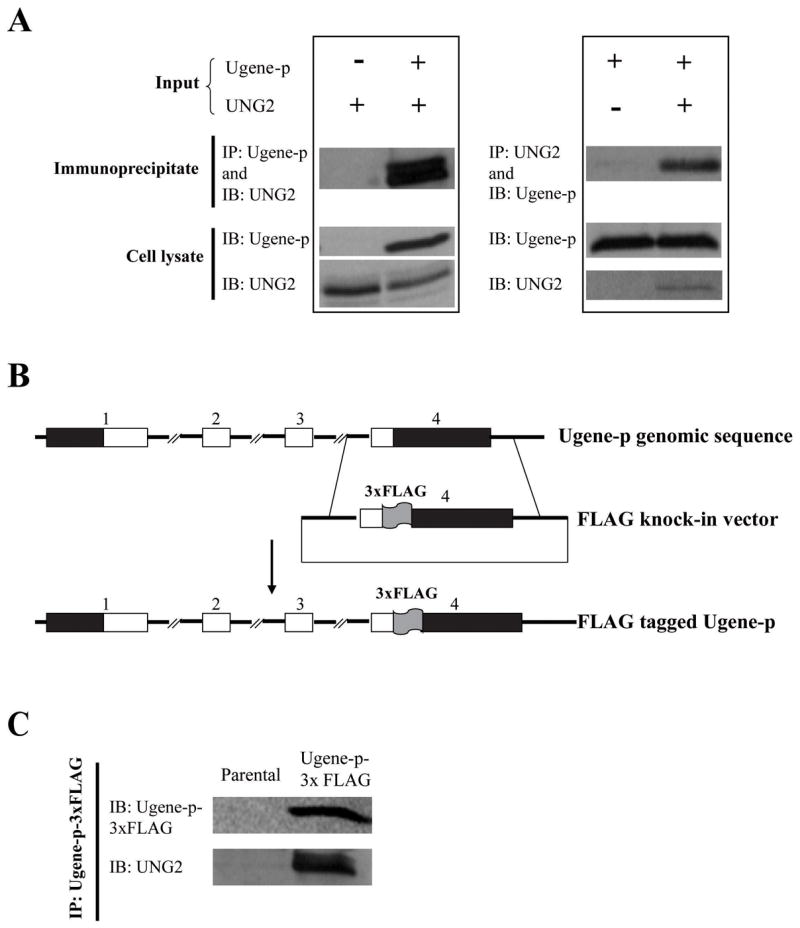
A) The left panel shows HEK 293 cells transfected with an expression vector for UNG2 (V5-epitope-tagged), and cotransfected with an expression vector for Ugene-p (FLAG-epitope-tagged) or the corresponding empty expression vector. Interaction of Ugene-p and UNG2 was tested by immunoprecipitation (IP) of Ugene-p and Western detection (IB) of UNG2 (Upper panel); expression of Ugene-p and UNG2 in the transfected cells is shown by Western blot against epitope tags (lower panel); The right panel shows HEK 293 cells transfected with an expression vector for Ugene-p (V5-epitope-tagged), and cotransfected with an expression vector for UNG2 (FLAG-epitope-tagged) or the corresponding empty expression vector. The upper panel again tests for interaction of Ugene-p and UNG2 by IP of UNG2 and Western detection of Ugene-p; the lower panel shows expression of translated proteins. B) Schematic diagram of the targeting vector for tagging endogenous Ugene-p with 3xFLAG. The numbers above the boxes denote exons; black boxes denote un-translated regions (UTRs); white boxes denote coding sequences. C) Western detection of endogenous Ugene-p and its interaction with endogenous UNG2. Ugene-p was immunoprecipitated from Ugene-p-3xFLAG tagged cells using FLAG antibodies. The upper panel demonstrates endogenous Ugene-p protein detected by Western blot (IB) against the FLAG epitope; the lower panel demonstrates the presence of co-immunoprecipitated UNG2 by Western blot against UNG2. Assay of parental DLD1 cells is shown as controls.
