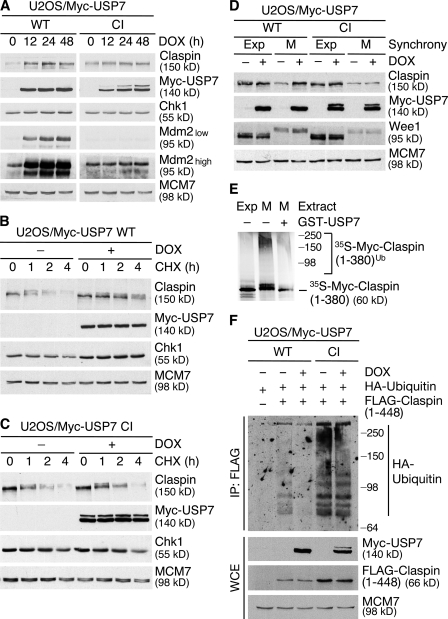
Figure 2.
USP7 deubiquitylates and stabilizes Claspin. (A) U2OS/Myc-USP7 cell lines were induced with doxycycline (DOX), harvested at the indicated times, and processed for IB with the indicated antibodies. (B) U2OS/Myc-USP7 WT cells were induced or not induced with DOX for 30 h, after which cycloheximide (CHX) was added to the cultures for the indicated times. The half-life of Claspin was estimated by IB of total cell extracts. (C) U2OS/Myc-USP7 CI cells were treated as in B. (D) U2OS/Myc-USP7 WT or CI cells were induced or not induced for 18 h with DOX, and left untreated (Exp) or incubated with nocodazole for an additional 12 h to synchronize cells in mitosis (M). The cell extracts were then analyzed by IB. Mobility-shifted Wee1 served as a marker for mitotically synchronized cells. (E) [35S]-labeled Claspin (amino acids 1–380) was incubated in ubiquitylation reaction mix, then supplemented with extracts of exponentially growing or mitotic U2OS cells and in vitro–translated βTrCP1. Where indicated, bacterially purified GST-USP7 WT was added to the reaction. Claspin ubiquitylation was visualized by autoradiography. Numbers to the right of the gel blots indicate molecular mass standards in kD. (F) U2OS/Myc-USP7 cell lines were transfected with indicated constructs for 24 h, and lysates were processed for IP with FLAG antibody and IB.