Scheme 13a.
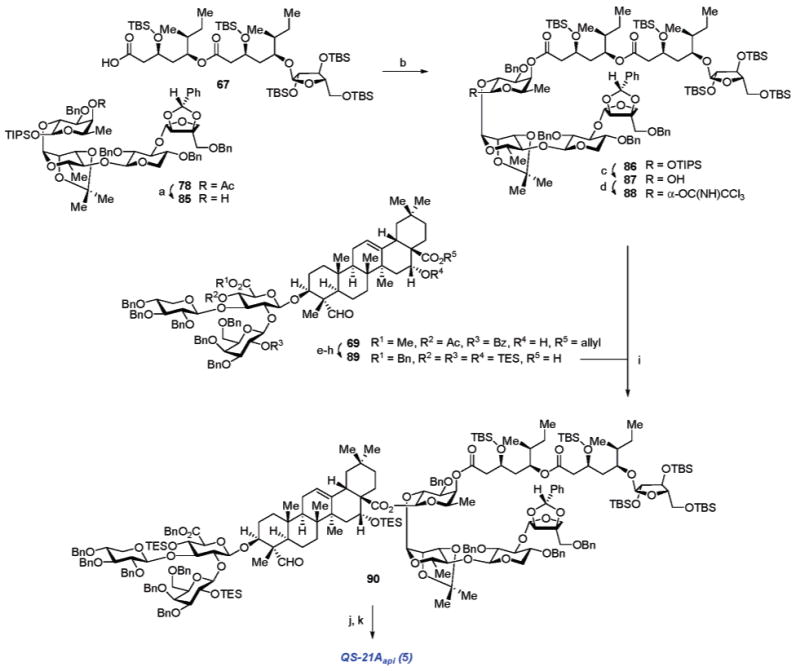
a Reagents and conditions: (a) K2CO3, MeOH; (b) 67, 2,4,6-C6H2Cl3COCl, Et3N, PhMe; 85, DMAP (90%, 2 steps); (c) TBAF, THF (81%); (d) CCl3CN, DBU, CH2Cl2 (56% α, plus 40% recovered 87); (e) NaOH, 1,4-dioxane; then Cs2CO3, H2O, MeOH; (f) KHCO3, BnBr, DMF (92%, 2 steps); (g) TESOTf, 2,6-lutidine, CH2Cl2; (h) HCO2H, Pd(OAc)2, Et3N, PPh3, 1,4-dioxane (81%, 2 steps); (i) BF3·OEt2, CH2Cl2 (70%); (j) TFA, H2O, CH2Cl2, 0 °C; (k) 150 psi H2, Pd/C, THF, MeOH (75%, 2 steps).
