Figure 3.
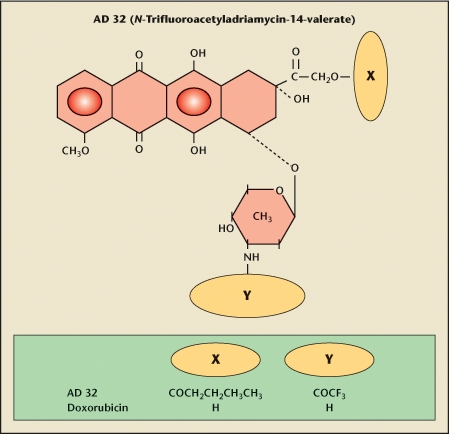
Valrubicin (AD32, Valstar) is N-trifluoroacetyladriamycin-14-valerate. It is a lipid-soluble anthracycline semisynthetic analogue of doxorubicin. The molecule is highly lipophilic and not very water soluble because of substitutions of the 14-carbon side chain of the valerate ester and the trifluoroacetyl group on the 3-amino group. Accordingly, valrubicin does not bind preferentially to the negatively charged cell membrane of hydrophilic agents like doxorubicin. This is the source of the decreased toxicity of valrubicin, and conversely, the source of the cardiotoxicity and contact toxicity of doxorubicin. Unlike doxorubicin, valrubicin rapidly traverses cell membranes and accumulates in the cytoplasm, where it interferes with the incorporation of nucleosides into the nucleic acids, resulting in chromosomal damage and cell-cycle arrest in G2.
