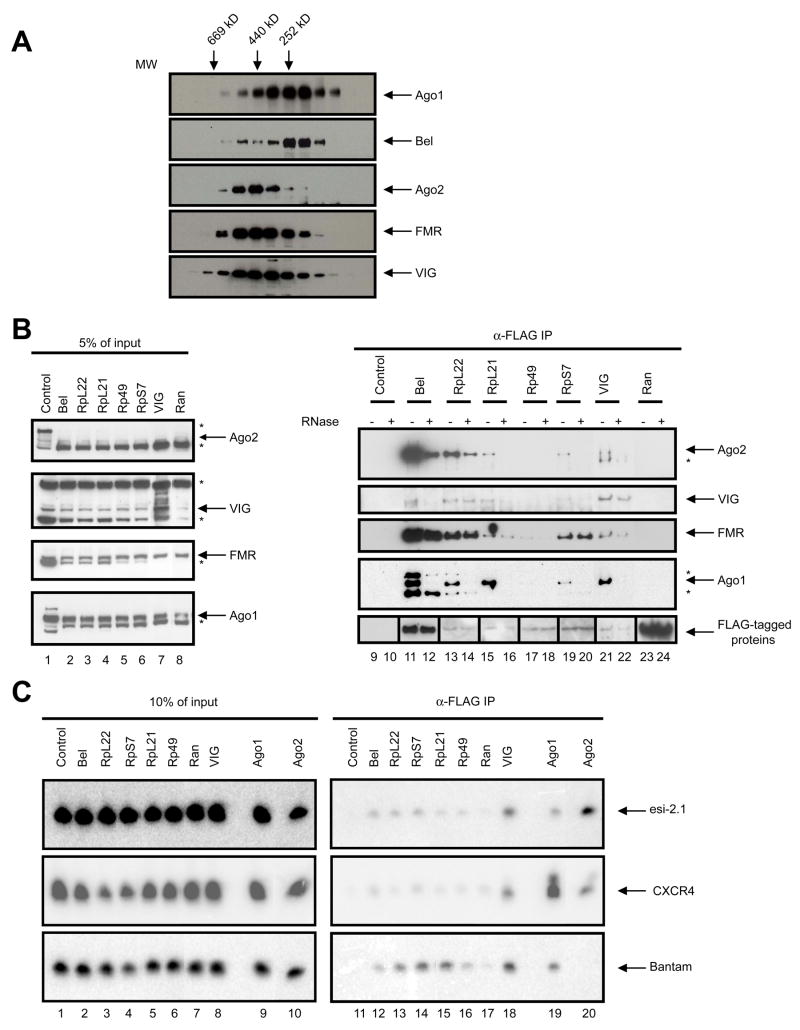
Fig. 4. Bel and RpL22 interact with components of the RISC.
(A) Cytoplasmic extract from S2-NP cells was fractionated by size exclusion chromatography, and the fractions were immunoblotted using antibodies against Bel and components of the RNAi pathway, as indicated. The elution profile of molecular markers is shown on top of the panel. (B) FLAG-tagged proteins (as labeled above the panel) were expressed in S2-NP cells and anti-FLAG immunoprecipitates were prepared. The samples were evenly split, and one set were treated with RNase while the other served as control. The samples were immunoblotted sequentially using antibodies against VIG, Ago2, FMR, Ago1 and FLAG. A small amount of cell extract was processed in parallel as input control. Non-specific bands are marked with a “*”. Note that the images shown in lanes 9 through 12 and 23 through 24 of the anti-FLAG panel were from films that were subjected to different exposure times from the rest due to differences in expression levels. (C) Small RNAs are enriched in the Bel and ribosomal protein immunoprecipitates. Expression constructs for FLAG-tagged proteins together with that for the CXCR4 siRNA mimetic were transfected into S2-NP cells. Total RNA was extracted from FLAG immunoprecipitates, and subjected to Northern blotting using probes against Bantam, esi-2.1 and CXCR4. To avoid signal saturation, approximately ~25% of the RNA recovered from the Ago1 and Ago2 samples was loaded compared to the rest of the samples. Total RNAs recovered from 10% of the cell extracts were processed in parallel as input control.