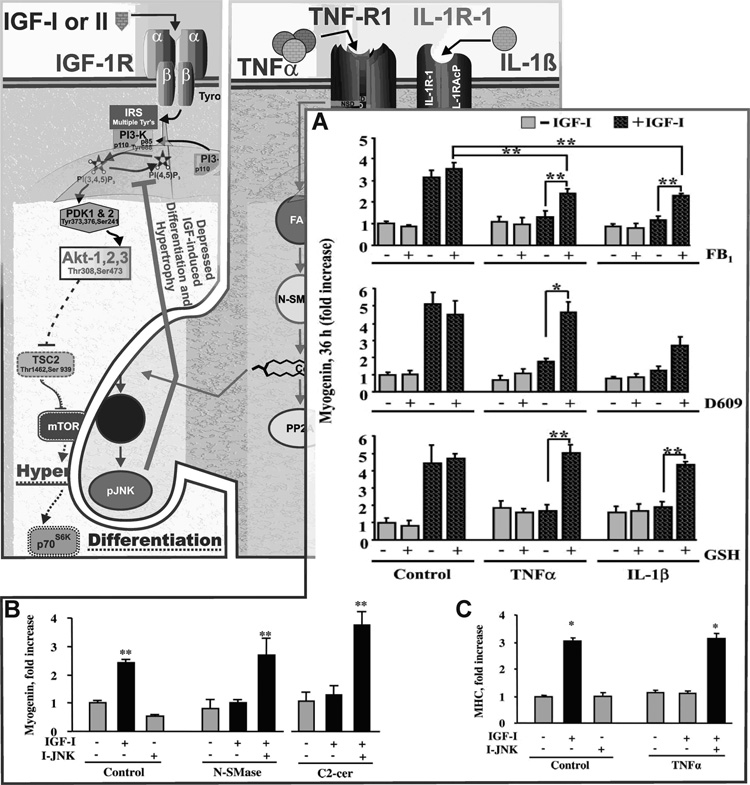
Fig. 4. Proinflammatory cytokines impair differentiation of C2C12 myoblasts only in the presence of IGF-I.
IGF-I increases both differentiation (myogenin expression; A and B) and hypertrophy (myosin heavy chain expression, MHC; C) of muscle cells. IGF-I increases differentiation in both a PI3-K- and Akt-dependent manner (not shown). TNFα and IL-1β depress IGF-I activity (both differentiation; A, and hypertrophy; C) by inducing IGF resistance. This resistance is associated with depressed activation of IRS-1. We have defined a pathway required for a low concentration of TNFα, 1 ng/ml, to inhibit IGF-I-induced myogenesis as follows: TNFα → N-SMase/A-SMase/ceramide synthase → ceramide → JNK → ↓ IGF effect by showing first that: A) inhibition of ceramide synthesis (de novo with FB1; A-SMase with D609 or N-SMase with GSH) blocks cytokine inhibition of IGF activity. Second, B) addition of N-SMase or a ceramide-derivative, C2-cer, mimic action of the cytokine. Third, B and C) the IGF resistance-inducing actions of N-SMase, C2-cer and TNFα are all blocked by a JNK inhibitor. Data shown with permission Endocrinology 145 (2004) : 4592–4602 and 147 (2006) : 4363–4373.