Table 1.
Members of the IGF System
| Ligands | F.W. | Structure | Primary Role | Location : source |
|---|---|---|---|---|
| IGF-I and IGF-II (70a.a. and 67a.a.) |
7.6 | Monomeric active forms | Drive metabolism, proliferation, survival, differentiation | All body fluids : all tissues |
| Insulin | 5.8 | Monomeric active form | Drives nutrient metabolism for storage | Circulation / pancreas |
| Receptors | Relative Affinities | Location | ||
| IGF-1R | 304 | Heterotetramer 2 α / 2 β | IGF-I > IGF-II >> Insulin 1 : 2 : 200 | One or both present on most, if not all, cells of the body |
| InsR (a/b isoforms) | 307 | Heterotetramer 2 α / 2 β | Insulin ≥ IGF-II > IGF-I 1 : 2 : 30 | |
| Natural Antagonists | Relative Affinities | Location : source | ||
| IGFBPs (1–6) | 25–30 | Monomers active | IGF-II ≥ IGF-I ≠ insulin | Body fluids, cell surfaces : all tissues |
| IGF-2R | 270 | Dimer | IGF-II >>>> IGF-I ≠ insulin | |
| sIGF-2R | 250 | Dimer | IGF-II >>>> IGF-I ≠ insulin | |
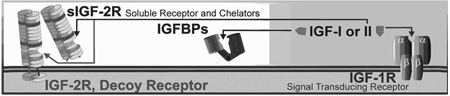 | ||||
IGF activity is controlled by a variety of mechanisms. Usually 5,000–20,000 hormone receptors are present on cells. Combined concentrations of the two IGF ligands in circulation are generally above 25 nM (~ 200 ng/ml) for mammals, which is much higher than that of insulin. Humans generally have higher circulating concentrations of IGFs. For example, 25–34-year-old adult humans have ~250 ng/ml (~33 nM) IGF-I in blood, which declines > 30% over the next 2 decades [166]. IGF-II in the serum of young (28-yr-old) adult humans is ~1200 ng/ml (~160 nM)[167]. These levels are much greater than the Kd (<1 nM) of the IGF-1R. Without additional modes of regulation, cells would be continuously activated by the IGFs. In contrast, insulin levels are below the Kd of its receptor but insulin concentrations rise toward the receptor Kd after a meal (example; insulin is < 0.1 nM = 0.58 ng/ml during fasting but rise to levels near the receptor Kd with feeding [168]). Thus insulin activity is primarily controlled extracellularly by changes in ligand concentration, however, control of IGF-1R activation is maintained by other specific extracellular proteins; 1) A signal deficient decoy receptor, the IGF-2R, which binds and prevents IGF-II from activating the IGF-1R. and 2) Six high-affinity, and several low affinity, IGFBPs that bind both ligands. Together, these binding proteins serve as direct extracellular negative regulators that maintain circulating ‘free’ IGF-I levels at or below 1 nM. At this concentration, small changes in free IGF result in proportionate changes in IGF-1R association and intracellular signaling. Each component within this system is independently regulated at the transcriptional level, which is particularly relevant to IGF-I and the IGFBPs whose extracellular levels are finely adjusted by a variety of inputs. An additional level of control exists for both the IGFs and insulin within the cell, where signaling from two or more factors can interact in either agonistic or antagonistic modes.
Sizes are approximate and based on amino acid composition of the mature protein (Swiss-Prot; ExPASy). Human and mouse IGF-I have 70 amino acids in the mature peptide; with only 4 non-conserved residues. IGF-I and IGF-II have high (>90 %) sequence conservation between the ligands of various species, human IGF-I and IGF-II are used for most studies since they react with the receptors of other species
