Figure 4.
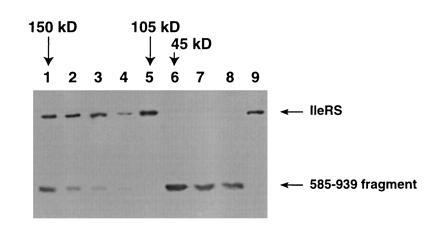
Gel filtration chromatography of inhibited complex. Full-length IleRS was denatured in 6 M guanidinium HCl in the presence of a 12-fold excess of the 585–939 fragment. The sample was renatured, and aminoacylation activity was found to be abolished. The sample was applied to a Sephacryl S300 gel filtration column (120 ml bed volume) in 50 mM potassium phosphate (pH 7.5), 0.1 mM NaCl, 50 mM 2-mercaptoethanol. Peaks of absorbance at 280 nm were analyzed by Western blotting with anti-IleRS antibody. Lanes 1–7 show fractions eluting from the gel filtration column. Lane 8, fragment 585–939 of isoleucyl-tRNA synthetase; lane 9, pure isoleucyl-tRNA synthetase. The retention times of protein standards are indicated by arrows above the figure. Protein standards for the column were: alcohol dehydrogenase (150 kDa; elution volume = 33 ml), IleRS (105 kDa; elution volume = 39 ml), and ovalbumin (45 kDa; elution volume = 46 ml).
