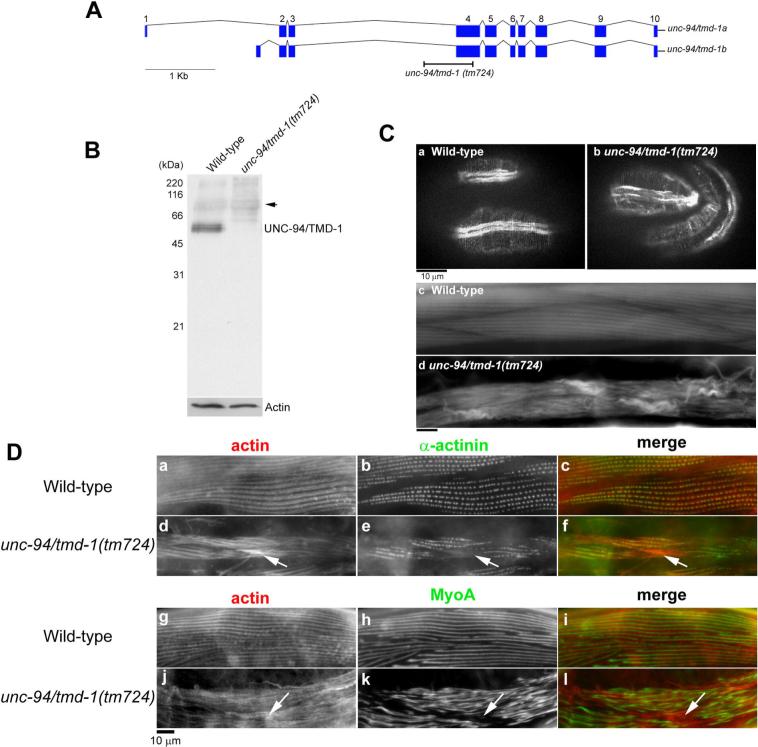
Figure 1.
An unc-94/tmd-1 mutation causes severe disorganization of actin filaments in body wall muscle. (A) Genomic organization of the unc-94/tmd-1 gene. Exons are indicated by boxes. Two alternatively spliced isoforms are generated as described (Stevenson et al., 2007). Position of the unc-94/tmd-1(tm724) deletion is indicated. (B) Western blot analysis of the TMD-1 protein in wild-type and unc-94/tmd-1(tm724) adult worms. The bands at approximately 68 kDa (arrow) are not related to the TMD-1 protein (Stevenson et al., 2007). Anti-actin antibody was used to monitor equal loading of the protein samples. (C) Actin filaments in embryos (a and b) and adults (c and d) were visualized by staining with tetramethylrhodamine-phalloidin in wild-type (a and c) and unc-94/tmd-1(tm724) (b and d). Bar, 10 μm. (D) Organization of α-actinin (a-f) and myosin (g-l) in body wall muscle. Wild-type (a-c and g-i) or unc-94/tmd-1(tm724) (d-f and j-l) were immunostained for actin (a, d, g, and j) and α-actinin (b and e) or MyoA myosin heavy chain (h and k). Merged images are shown in c, f, i, and l (actin in red and α-actinin or MyoA in green). Arrows indicate positions of actin aggregates where α-actinin or myosin did not localize. Bar, 10 μm.