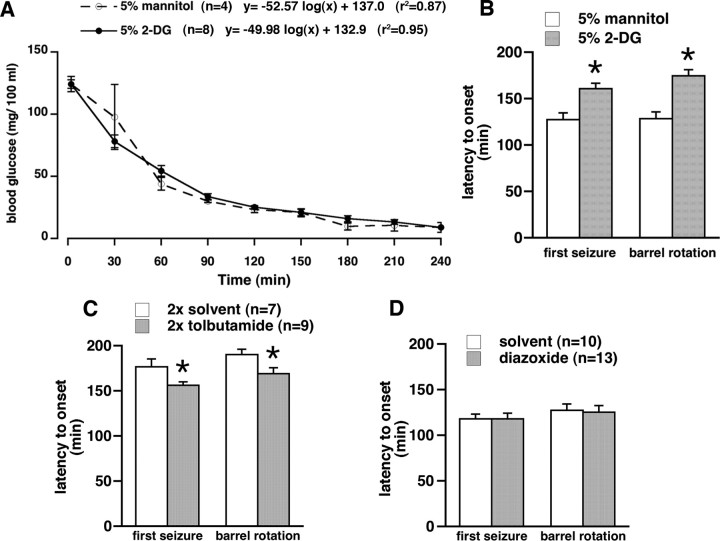
Figure 6.
Effects of drug microinfusions in the SNR on hypoglycemic seizures. A, Decrease of blood glucose with time after insulin injection in the two groups of nonfasted rats used for the experiment illustrated in B. There was no difference between the groups. There was a very good logarithmic fit, almost identical to the curve fit for the nonfasted rats in the Figure 1. B, Microinfusion of nonradioactive 2-DG, a glucose anti-metabolite, in the anterior part of the SNR (0.5 μl of the 5% solution per site), significantly delayed onset of first seizure and barrel rotation after insulin injection compared with rats microinjected with the same volume of 5% mannitol (mean ± SEM; *p < 0.05, t test). This finding is consistent with opening of postsynaptic KATP channels because of acute ATP deficiency resulting in hyperpolarization and decreased firing of the SNR neurons, and therefore, with anticonvulsant effects (Velíšková and Moshé, 2006). C, Double microinfusion of tolbutamide, a KATP channel blocker, in the anterior part of the SNR (0.25 μl of the 4 mm solution) of nonfasted rats at 24 h and immediately before insulin significantly accelerated onset of first seizure and barrel rotation compared with rats microinfused with solvent (DMSO/β-cyclodextrine mixture, 5:1 ratio; mean ± SEM; *p < 0.05, t test). This finding is consistent with closing of postsynaptic KATP channels resulting in depolarization and increased firing of the SNR neurons and, thus, proconvulsant effects (Velíšková and Moshé, 2006). D, Microinfusion of diazoxide, a KATP channel opener, in the anterior part of the SNR (0.25 μl of the 4 mm solution) of fasted rats did not change susceptibility to development of hypoglycemic seizures compared with rats microinfused with solvent (0.1N NaOH plus PBS), indicating that the effector system (postsynaptic KATP channels) may have been unavailable as suggested by decreased Kir6.2 subunit expression in fasted rats (Fig. 5).