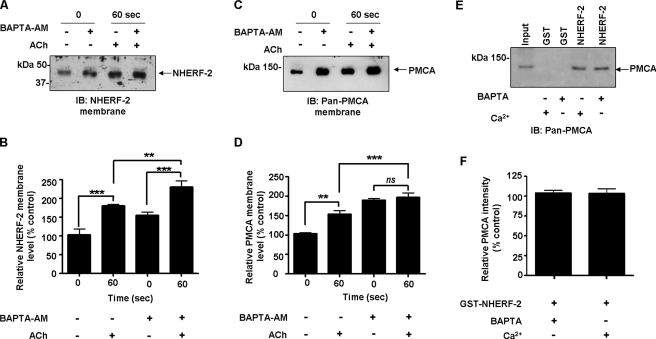
FIGURE 9.
Chelation of intracellular Ca2+ alters ACh-evoked trafficking of PMCA and NHERF-2, but does not alter the association of the proteins in vitro. HT-29 cells were preincubated with BAPTA-AM (50 μm), exposed to ACh (10 μm), and membrane-associated NHERF-2 and PMCA detected. Whole cell lysates were incubated with BAPTA and GST pull-down experiments performed with NHERF-2 to evaluate if the association between NHERF-2 and PMCA was Ca2+-dependent. A, representative Western blot of membrane-associated NHERF-2 ± BAPTA-AM and ACh. B, densitometry of membrane-associated NHERF-2. Under control conditions, NHERF-2 increased by 80 ± 6% over control after 60 s of exposure to ACh (n = 3; ***, p < 0.001). When Ca2+ was chelated with BAPTA-AM, there was a significant increase in NHERF-2 to 54 ± 5% over control unstimulated cells (n = 3; **, p < 0.01). Addition of ACh resulted in a further significant increase in membrane-associated NHERF-2 after 60 s to 130 ± 16% (n = 3; **, p < 0.001). C, representative Western blot of cell surface-associated PMCA ± BAPTA-AM and ACh. D, densitometry of membrane-associated PMCA. Under control conditions, PMCA increased by 53 ± 5% over control after 60 s of exposure to ACh (n = 3; ***, p < 0.001). When Ca2+ was chelated with BAPTA-AM, there was a significant increase in membrane-associated PMCA to 89 ± 5% above control (n = 3; ***, p < 0.001). Addition of ACh failed to increase the levels of membrane-associated PMCA in BAPTA-AM-treated cells. E, representative Western blot of GST-NHERF-2 pull-downs in HT-29 cells in the absence of Ca2+. F, densitometry of relative PMCA intensity for GST pull-down in vitro binding assay with NHERF-2. The association between NHERF-2 and PMCA was not altered when Ca2+ was chelated. All data are expressed as mean ± S.E.