FIGURE 1.
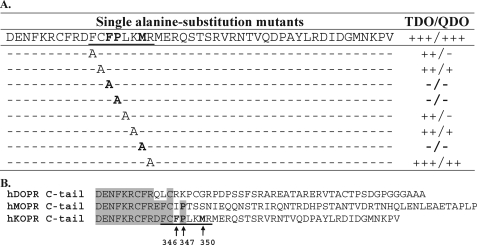
Mapping the GEC1-binding motif in the C-tail of hKOPR using Y2H assays. A, summary of Y2H results. Truncation and double and single alanine substitution mutants of hKOPR C-tail were examined for their abilities to interact with GEC1-(38–117). The hKOPR C-tail mutants were generated by PCR, constructed into GAL4-binding domain vector pGBKT7, and transformed into yeast strain AH109. The GEC1-(38–117) was constructed into GAL4 activation domain vector pGADT7 and transformed into yeast strain Y187. The two transformed yeast strains mated, and the mating mixtures were cultured on SD/-Trp/-Leu (2DO), SD/-Trp/-Leu/-His (TDO), and SD/-Trp/-Leu/-His/-Ade (QDO) agar plates. The resulting diploid colonies were counted, and the ratio of the number of colony-forming units on TDO/QDO plates over colony-forming units on the 2DO plate was used to evaluate the interaction strength of each pair of GEC1-(38–117) and hKOPR C-tail mutant. Assessment details were described under “Experimental Procedures.” The experiments were performed three times with reproducible results. Results on truncation and double alanine substitution mutants are shown in supplemental Fig. 1. B, amino acid sequence alignment of C-tails of the human opioid receptors demonstrating that the defined GEC1 binding region is hKOPR-specific.