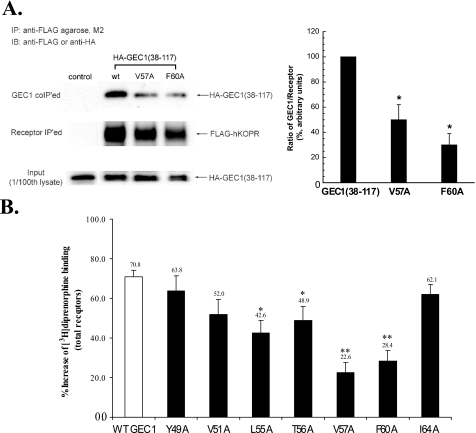
FIGURE 5.
A, V57A or F60A mutation in GEC1-(38–117) GEC1 reduced its co-immunoprecipitation with FLAG-hKOPR. Wild-type GEC1-(38–117) or its mutant was co-transfected into CHO cells with wild-type FLAG-hKOPR or the control vector. The receptors were immunoprecipitated (IP) using anti-FLAG (M2)-agarose beads 40 h after transfection. SDS-PAGE was performed, and GEC1-(38–117) and the mutants (GEC1 co-IPed, 1st row) and FLAG-hKOPR (receptor IPed, 2nd row) were detected with immunoblotting (IB). The ratios of density of GEC1-(38–117) or the mutants over FLAG-hKOPR were normalized against that of wild-type GEC1-(38–117) and are shown in the graph at right. Each value represents the mean ± S.E. of three independent experiments. *, p < 0.05 compared with wild-type group using one-way ANOVA followed by Tukey's post hoc test. B, effects of single alanine substitution of the critical residues in the hKOPR-binding motif on the GEC1-induced enhancement in FLAG-hKOPR expression. Wild-type GEC1 and its alanine-substituted mutants were cloned in pcDNA3.1 and then transiently transfected into CHO cells stably expressing FLAG-hKOPR. Forty hours after transfection, [3H]diprenorphine (1 nm) binding to hKOPR was performed on intact cells. Data were expressed as the percentage of increase in [3H]diprenorphine binding in GEC1-transfected cells compared with the control cells that were transfected with the vector pcDNA3.1. Each value represents the mean ± S.E. of five independent experiments. *, p < 0.05, and **, p < 0.01 compared with wild-type group using one-way ANOVA followed by Tukey's post hoc test.