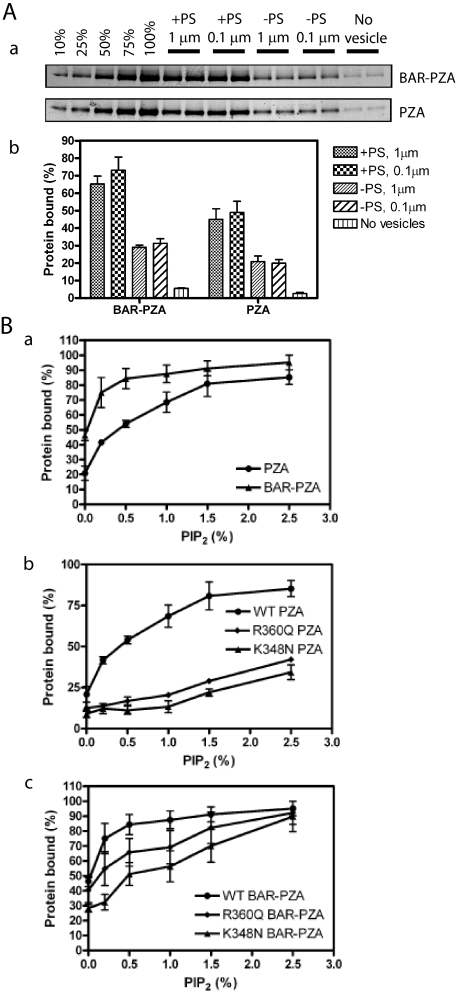
FIGURE 3.
Membrane binding of ASAP1. A, contribution of the BAR domain to membrane association. BAR-PZA (0.7 μm) and PZA (1.2 μm) were incubated with 500 μm sucrose-loaded LUVs composed of either 40% PC, 25% PE, 15% PS, 9.5% PI, 0.5% PIP2, and 10% cholesterol (+PS) or 55% PC, 25% PE, 9.5% PI, 0.5% PIP2, and 10% cholesterol (-PS) and formed by extrusion through either a 1- or 0.1-μm pore filter. Vesicles were precipitated by centrifugation, and associated proteins were separated by SDS-PAGE. The amount of precipitated protein was determined by densitometry of the Coomassie Blue-stained gels with standards on each gel. Panel a, representative experiment. The raw data are shown from one experiment representative of three. The first five lanes are standards added to the gel to quantify the amount of protein that was precipitated with the vesicles. The numbers are the percentage of the total protein used for the binding experiment. Panel b, summary of three experiments. Averages and S.E. are presented in the figure. B, contribution of the PH domain/phosphatidylinositol 4,5-bisphosphate association for binding to LUVs. Panel a, comparison of PIP2-dependent binding of BAR-PZA and PZA. The experiment was performed as described in A but with +PS LUVs containing the indicated concentrations of PIP2. Data are the average and S.E. from three experiments. Panel b, effect of mutation of the PH domain on PIP2-dependent binding to PZA to LUVs. The experiment was performed as described in A but using the indicated recombinant ASAP1. Data are the average and S.E. of three experiments. Panel c, effect of mutation of the PH domain on PIP2-dependent binding of the BAR-PZA to LUVs. The data are the average and S.E. of three experiments. WT, wild type.