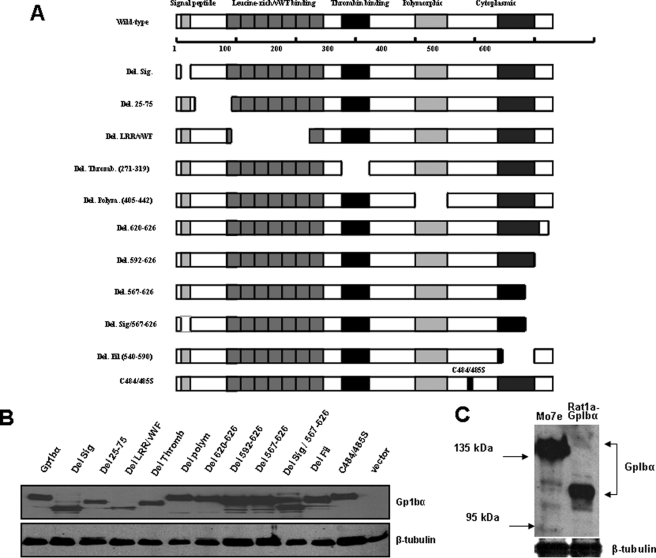
FIGURE 2.
Expression of human GpIbα mutants. A, schematic diagram depicting all of the mutants listed in Table S3. The top shows the full-length, 627-amino acid GpIbα protein and several of the domains known to be relevant for its function as a subunit of the vWFR. In platelets, the signal peptide is typically cleaved to yield a mature protein of 611 amino acids. However, the numbering of the protein here includes the signal peptide. Note that the cytoplasmic domain comprises two previously described functional elements; these comprise a membrane-proximal filamin-binding domain and a 6-amino acid C-terminal tail involved in vWRF-related signaling. All mutants were generated in the pLXSN-EYFP retroviral vector and were stably expressed as C-terminal c-Myc epitope-tagged proteins in Rat1a cells. B, immunoblotting of Rat1a cells stably expressing each of the mutants depicted in A. Comparable amounts of whole cell extracts were resolved by SDS-PAGE and processed for Western blotting, as described previously (16). The upper portion of the blot was probed with a mAb specific for the c-Myc epitope tag, and the bottom portion was probed with a mAb specific for β-tubulin. C, the molecular mass of GpIbα in transfected Rat1a cells was compared with that of endogenous GpIbα in Mo7e megakaryoyte cells, which express an intact vWFR (27). The blot was probed with the WM23 mAb that is specific for human GpIbα. Note that the latter protein possessed an apparent molecular mass significantly higher than that of the former (∼130–140 kDa versus ∼110–115 kDa) as a result of its more extensive post-translational glycosylation (29–32).