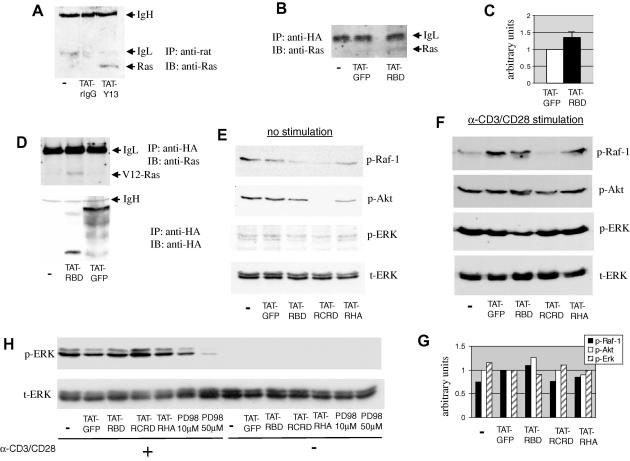
Fig. 3.
Interaction of TAT proteins with endogenous Ras and inhibition of the homeostatic, but not the anti-CD3/CD28-induced, phosphorylation of Ras effectors. Jurkat cells were left untreated (−) or incubated with 20 μg/ml TAT-linked mAbs (A) or TAT-fusion proteins (B) for 90 min. In (A) internalized Abs were immunoprecipitated with anti-rat IgG, while in (B) cells were immunoprecipitated with anti-HA mAb. Co-immunoprecipitated endogenous Ras was detected by Western blotting. Representative data are shown from three similar observations. IgH and IgL denote the heavy and light chain of the immunoprecipitating antibody, respectively. (C) Cumulative data from three experiments of the intensity of the bands that correspond to co-immunoprecipitated Ras from cells incubated with TAT-GFP and TAT-RBD. P < 0.06. (D) COS-7 cells expressing the G12V-Ras mutant were incubated with 20 μg/ml of the indicated TAT-fusion proteins and internalized recombinant proteins were precipitated with anti-HA. Co-immunoprecipitated Ras was detected by Western blotting. Splenocytes cultured with 20 μg/ml of the indicated TAT-fusion proteins were left unstimulated (E) or were stimulated with anti-CD3/CD28 coated beads for 5 min (F). Equivalent amount of lysates were sequentially immunoblotted with antibodies specific for the phosphorylated/activated forms of c-Raf-1, Akt, and ERK1/2. Protein loading was assessed with an anti-ERK2 antibody. (G) Densitometric analysis of the blots shown in (F) normalized for protein loading according to the intensity of the bands in the t-ERK blot. Results are expressed in arbitrary units in which the TAT-GFP treatment control was set at the value of 1. (H) Jurkat cells were incubated with 20 μg/ml of TAT-fusion proteins and then stimulated for 5 min with anti-CD3/CD28 antibodies. ERK1/2 phosphorylation was determined as above. p-ERK, phosphorylated ERK; t-ERK, total ERK; PD98, MEK inhibitor PD098059. The blots in panel (E) were exposed considerably longer compared to blots in (F) and (G).