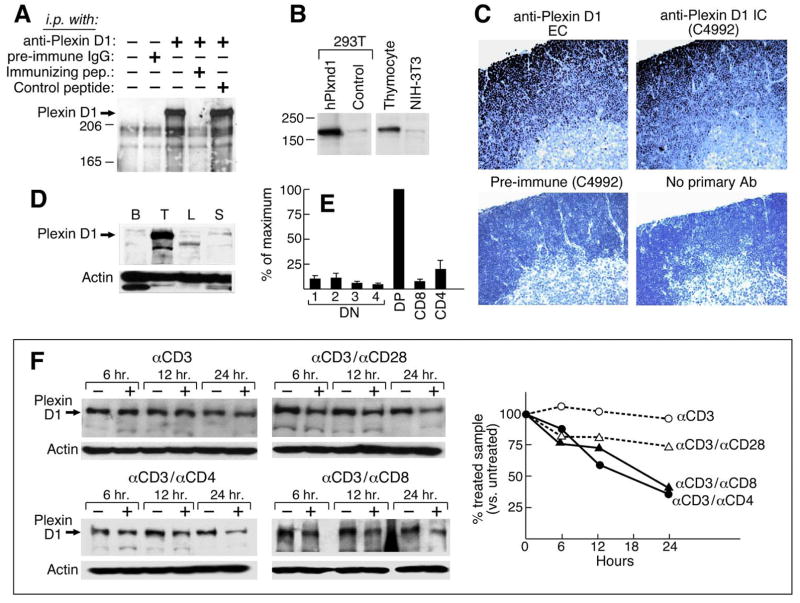
Figure 2. Expression and regulation of plexin D1 on thymocytes.
A. A lysate of surface biotinylated thymocytes from 3–4 week old B6 mice was prepared. Following preclearing with goat IgG agarose, samples representing 2 × 107 cells were incubated with preimmune Ab, affinity-purified anti-plexin D1 IC or with the same Ab plus a 150-fold molar excess of the immunizing peptide or an irrelevant control peptide. After SDS/PAGE and transfer, the membrane was probed with steptavidin-HRP. B. Western blotting using affinity-purified anti-plexin D1 IC on lysates of 293T cells transfected with human plexin D1 cDNA, 293-T cells transfected with an irrelevant control cDNA, primary thymocytes, and NIH3T3 mouse fibroblasts separated by SDS-PAGE (8%). C. Paraffin sections were stained with indicated anti-plexin D1 EC, IC or preimmune serum as a control. Sections were counterstained with Mayer’s hematoxylin. D. Single cell suspensions were prepared from the indicated organs of C57BL/6 mice. Total cell lysates were resolved on 6% SDS-PAGE and blotted with anti-plexin D1 IC and anti-actin antiserum (B: bone marrow; T: thymus; L: lymph node; S: spleen). E. Total RNAs were isolated from sorted thymocyte populations and used for cDNA synthesis. Synthesized cDNA was used for Realtime PCR analysis according to the manufacturer’s instructions (ABI). Normalized values using GAPDH real time PCR values with the same cDNA as a control were converted to % of maximum value (value of DP thymocyte population). Error bars are the standard error of 3 independent experiments. F. Single cell suspensions of B6 thymocytes were prepared and then stimulated with the indicated combinations of mAbs for 6, 12 and 24 h. Total cell extracts were prepared from stimulated cells after removal of dead cells using Histopaq (Sigma, MO) and analyzed by western blot with anti-plexin D1 IC. This figure is representative of 3 independent experiments. The graph shows quantitated-plexin D1 band intensities normalized as % of untreated sample.