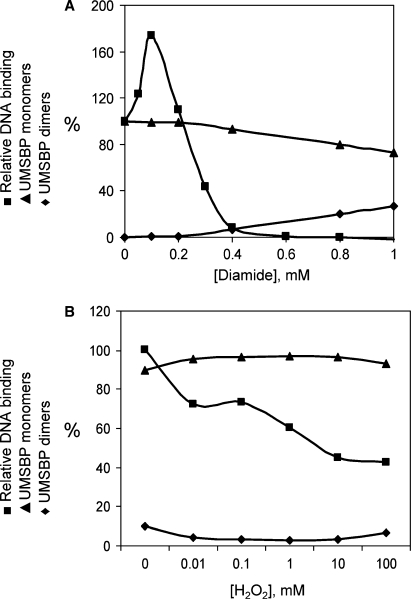
Figure 2.
Inhibition of UMSBP binding to DNA by oxidation is not mediated by the protein oligomerization. The effect of oxidation on UMSBP DNA-binding activity and its oligomerization was measured in vitro (A) and in vivo (B). In (A), UMSBP was incubated in the presence of the indicated diamide concentrations. DNA binding was measured using EMSA with UMS ligand and UMSBP oligomerization using SDS–PAGE under non-reducing conditions. Squares, DNA-binding activity; diamonds, UMSBP oligomers; triangles, UMSBP monomers. In (B), C. fasciculata cell culture was treated with the indicated concentrations of H2O2 and cell lysates were assayed for UMS-binding activity and UMSBP oligomerization. Squares, UMSBP DNA-binding activity; diamonds, UMSBP oligomers; triangles, UMSBP monomers. UMS-binding activity is presented relative to the activity of untreated UMSBP, used as a 100%. The sum of the monomer and oligomer fractions are used as a 100% in each oxidation point and their relative abundance (%) is presented.