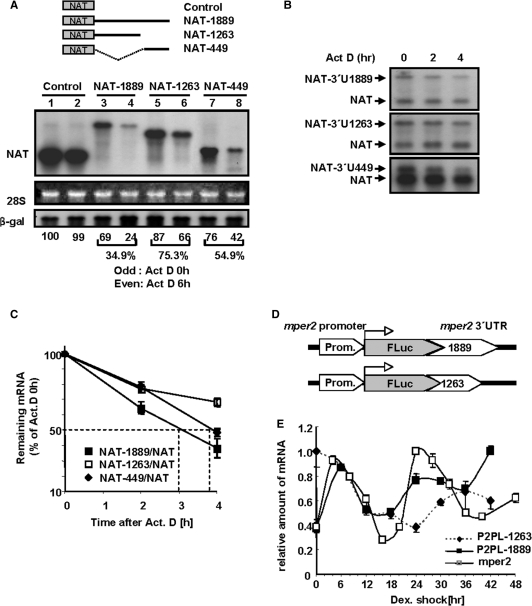
Figure 2.
mper2 3′-UTR is responsible for its own mRNA degradation.(A) Northern blot analysis of NAT reporter: 3′-UTR constructs. CHO-K1 cells were transiently transfected by electroporation with NAT reporter alone or NAT reporter fused to serially deleted mper2 3′-UTR (shown in schematic diagrams, the numbers indicate nucleotide lengths of the 3′-UTR). β-gal was used as a transfection control. Transfected cells were incubated for 12 h before treatment with 5 μM actinomycin D. Total RNA (10 μg) was hybridized with a probe specific for AANAT coding sequence. Et-Br stained 28S rRNA provided a loading control. Odd numbered lanes, 0 h; even numbered lanes, after 6 h treatment with 5 μM actinomycin D. (B) Degradation kinetics and half-life of 3′-UTR:reporter constructs were analysed by actinomycin D pulse chasing and northern blot hybridization. Experimental schemes are the same as in panel A. Representative results of three independent experiments are shown. (C) The band intensities in panel B were quantified by phosphoimaging and normalized to the intensity of cotransfected AANAT control in each lane. The graph shows the mean ± SEM of three independent experiments. (D) Diagrams of recombined reporter constructs consisting of mper2 promoter (∼1.7 kb), firefly luciferase open reading frame, and either full-length mper2 3′UTR or 3′ region deleted mper2 UTR. (E) Firefly luciferase mRNA expression from stably recombined reporter vectors and endogenous mper2 mRNA were measured by quantitative real-time RT–PCR using cDNA harvested at 6 h (cell line) or 4 h (endogenous mper2) intervals from stably transfected NIH 3T3 cell lines that were synchronized by treatment with dexamethazone. All results are representative of three independent experiments from each selected line.