Figure 1.
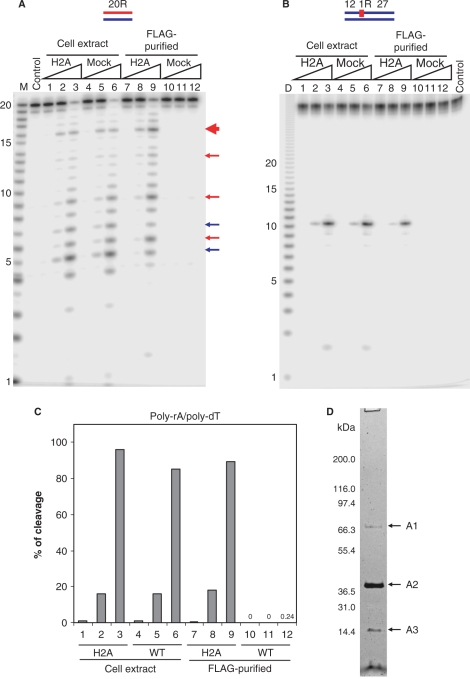
RNase H activity in cell extracts and FLAG-tag purified fractions from RNASEH2A expressing and untransduced HeLa cell lines. The 5′-end 32P-labeled 20-bp RNA/DNA hybrid (A) and DNA12–RNA1–DNA27/DNA40 hybrid (B) were cleaved with increasing amounts of the total cell extracts and FLAG-tag purified fractions from RNASEH2A expressing cells (H2A) and untransduced cells (mock) at 37°C for 15 min. The 500 μl cell extracts from 107 cells yielded 200 μl of FLAG-purified samples as described in Materials and methods section. Ten picomoles of substrates were treated with 1 μl of the samples in 10 μl of reaction mixtures. Protein samples were diluted in Dilution Buffer. Lanes 1, 4, 7 and 10 contained 0.002 μl equivalents of the undiluted sample, lanes 2, 5, 8 and 11 contained 0.02 μl equivalents and lanes 3, 6, 9 and 12 contained 0.2 μl equivalents. After digestion the reactions were electrophoresed in a 20% TBE-urea PAGE and the gel analyzed on a phosphoimager. Note (B) the mobilities of the DNA12 product of DNA12–RNA1–DNA27/DNA40 migrates faster than the RNA size markers due to inherent differences in migration in the gels between RNA and DNA. In (A), major cleavage sites of 20-bp RNA/DNA hybrid with RNase H1 and RNase H2 are indicated with blue and red arrows, respectively. The main cleavage product of RNase H2 is indicated by a thick red arrow. Molecular size markers are indicated as M (products of digestion of 32P-labeled 20-mer RNA by Phosphodiesterase I) (measuring the sites of cleavage from the 5′- label of the 20-mer RNA) and D (products of digestion of 32P uniformly labeled poly-rA/poly-dT by mouse RNase H1) (measuring the sizes of products that have uniform sequences). (C) Uniformly 32P-labeled poly-rA/poly-dT (1 μM) was cleaved with increasing amount of the total cell extracts and FLAG-tag purified fractions. Amounts of samples in lanes 1–12 are equivalent to those of (A). The ratios of cleavage products were determined by measuring the acid-soluble radioactivity. (D) SDS–PAGE of the purified RNase H2 from HeLa RNASEH2A cells with the two-step affinity immunopurification. HeLa RNASEH2A cells were extracted and subjected to anti-FLAG and anti-HA two-step purification. The purified sample was analyzed by SDS–PAGE stained with silver staining. The fragment indicated with A1, A2 and A3 were identified by mass spectrometry, as described in text.