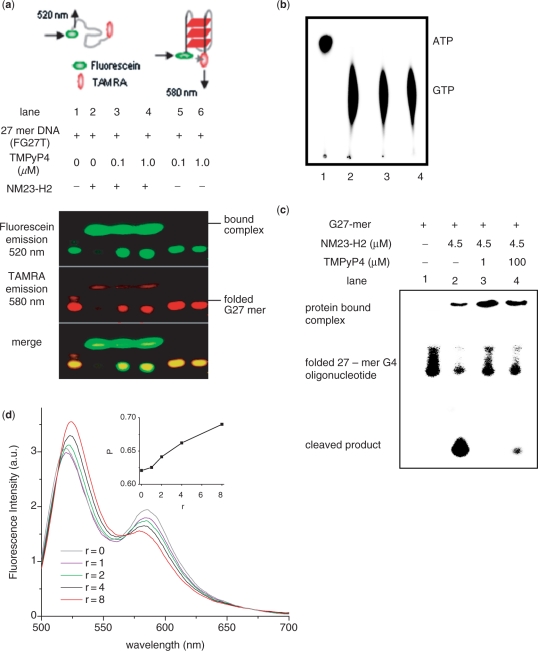
Figure 2.
NM23-H2 binds to G4 motif in vitro. (a) Electrophoretic mobility shift of 27-mer oligonucleotide (FG27T, see Methods) from c-MYC promoter NHE in presence or absence of NM23-H2 and/or TMPyP4 in 5% native PAGE; protein bands were independently stained by Coomassie (data not shown). Top panel: schematic representation of energy transfer in unfolded and folded (parallel G4 motif) along with respective predominant emission wavelengths. (b) Protein function (kinase) activity of NM23-H2 was not affected in presence of TMPyP4. NM23-H2-mediated phosphorylation of GTP using [γ-32P]ATP was assayed in presence of TMPyP4. Lane 1: [γ-32P]ATP alone; lane 2: with NM23-H2; lanes 3 and 4: with NM23-H2 and 60 μM (lane 3) or 120 μM (lane 4) TMPyP4. (c) Nuclease activity of NM23-H2 is inhibited in presence of TMPyP4. Ten-nanomolar radiolabeled (along with 0.1 μM unlabeled) 27-mer oligonucleotide sequence (5′-TGG GGAGGGTGGGGAGGGTGG GGAAGG-3′) that forms G4 motif (23) in the c-MYC promoter NHE was incubated with 4.5 μM NM23-H2, either alone (lane 2), or with 1 μM (lane 3) or 100 μM (lane 4) TMPyP4 in presence of NM23-H2 in buffer [10 mM HEPES, 2 mM MgCl2, 20 mM KCl, 50 µg/ml BSA, 0.5 mM DTT (pH 7.4)]; lane 1: oligonucleotide only as control. All reactions in (b) and (c) had 60 μM single-strand poly(dA) as nonspecific control and TMPyP4 treatments were for 45 min followed by 3 h incubation with protein at 37°C. (d) FRET (detected in solution) of FG27T (50 nM) as a function of increasing concentration of NM23-H2 (r = NM23-H2/FG27T) in 20 mM K+; spectra were recorded following a 10-min incubation after each addition. Inset: Change in energy transfer (represented as P, determined from experimental FRET data) as a function of increasing (r); P = ID/(ID+IA), where ID and IA are average donor (fluorescein) and acceptor (TAMRA) intensities, respectively. Experiments were done in triplicate and for each value of P error of ± 0.07 arbitrary units was observed.