Figure 1.
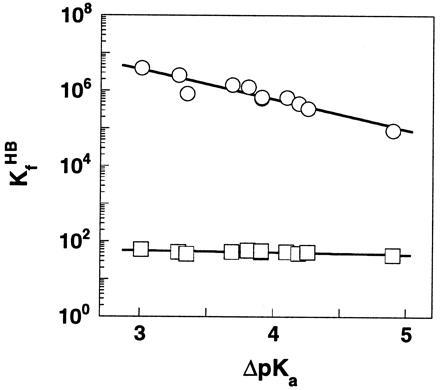
The dependence of log KHB upon ΔpKawater in DMSO (○) and H2O (□) for the H bond in substituted SA monoanions. The Brønsted slopes are 0.05 and 0.73 in water and DMSO, respectively. A common ΔpKa scale in water was used to allow a direct comparison of the magnitude of changes in H bond strength in the two solvents. Data are from Table 1.
