Figure 4.
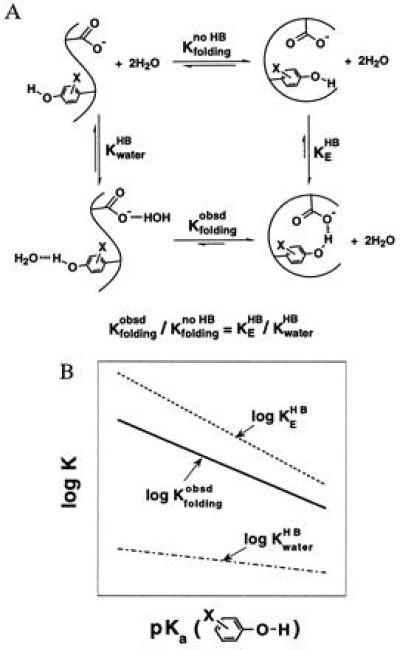
A large Brønsted slope for H bonding in proteins is inferred from folding studies of Staphylococcal nuclease mutants. (A) Thermodynamic analysis depicting the effect of changing the strength of the H bond donor, the substituted tyrosine hydroxyl, on the stability of the protein. The folding equilibrium of a hypothetical non-H-bonded species (Kfoldingno HB) is used to dissect the effects from H bonding. (B) Schematic depiction of the dependences of H bonding and folding equilibria on the pKa value of substituted tyrosines. As the tyrosine hydroxyl becomes more acidic, the strengthening of its H bond to the enzymatic glutamate and to water stabilizes the folded and unfolded protein, respectively. The slope of the plot of log Kfoldingobsd versus pKa is the difference between the slopes of plots of log KEHB versus pKa and KwaterHB versus pKa. This follows from the thermodynamic relationship shown in A, which gives Δlog Kfoldingobsd = Δlog KEHB − Δlog KwaterHB (i.e., the greater strengthening of the H bond on the protein results in a change in the observed stability of the protein. Note that Δlog Kfoldingno HB = 0 by definition because the folding equilibrium between the non-H-bonded species does not depend on the strength of the H bond donor.
