Figure 1.
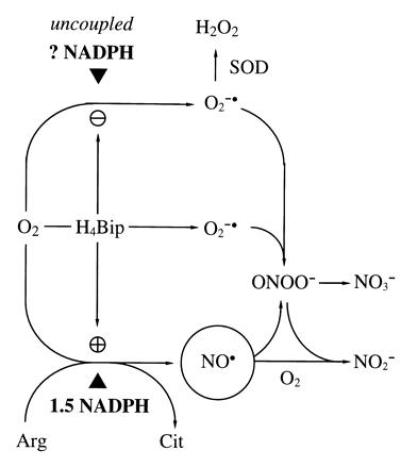
Prevailing hypothesis of the NOS reaction mechanism. O2 is reductively activated by NADPH and NOS to oxidize Arg (14) to the proximal NOS product, ·NO (circled). A fraction of total NADPH consumption and O2 activation is uncoupled from Arg turnover (15), more so in the absence of H4Bip (16), resulting in substantial O2⨪ formation (NADPH oxidase activity of NOS). O2⨪ limits detection of ·NO by a diffusion-limited reaction to ONOO−, which breaks down to NO3− (17, 18). Addition of H4Bip will further stimulate NOS activity and prevent uncoupling of NADPH consumption and O2 activation (16) but, due to its autoxidation in aerobic solutions, also provides an alternative source for O2⨪ (19, 20). Superoxide dismutase (SOD; EC 1.15.1.1) enables ·NO detection (19, 21), in the absence and presence of H4Bip, by dismutating O2⨪ to H2O2, which does not interfere with ·NO detection. In the absence of O2⨪, ·NO decays in a third-order reaction with O2 to yield NO2−. ▴ indicates required reducing equivalents—i.e., 1.5 mol of NADPH per mol of Cit. H4Bip is assumed to activate NOS allosterically but not to donate reducing equivalents.
