Figure 7.
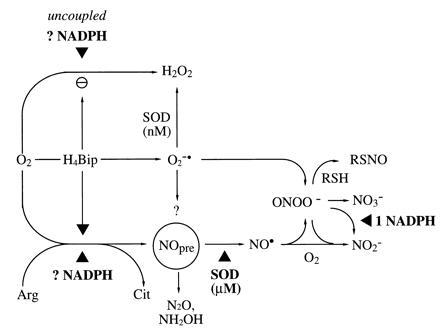
Modified hypothesis on the NOS reaction mechanism and interaction of NOS reaction products. In the absence of H4Bip, uncoupled NADPH consumption and O2 activation appears to yield primarily H2O2, not O2⨪. Thus, analytical limitations cannot explain the absence of a ·NO signal from NOS-catalyzed Arg turnover under these conditions. SOD converts a proximal NOS product, the ·NO precursor (NOpre), to ·NO. Candidate molecules for NOpre include NH2O· and NO−, yielding N2O and NH2OH as by-products. Generated ONOO− (e.g., from H4Bip-derived O2⨪ + ·NO or O2 + ·NO−) will, in a secondary reaction, consume additional NADPH equivalents. ONOO− can be scavenged by high thiol concentrations, providing an alternative source for RSNO, and yield not only NO3− but also NO2−. ▴ depicts requirement for redox equivalents (NADPH, SOD, and possibly also H4Bip).
