Figure 6.
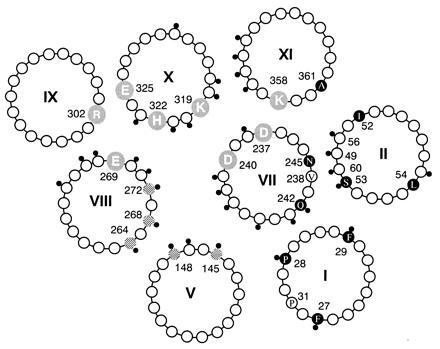
Packing of helices I, II, V, and VII–XI in the lac permease viewed from the periplasmic surface. The four essential residues (Glu-269, Arg-302, His-322, and Glu-325), two interacting pairs of Asp–Lys residues [Asp-237 (helix VII)/Lys-358 (helix XI), and Asp-240 (helix VII)/Lys-319 (helix X)] are highlighted. Positions of NEM-sensitive Cys replacements are indicated with a small black dot. Substrate protectable single Cys replacement mutants (145, 148, 264, 268, and 272) are crosshatched. Cys replacement mutants tested for disulfide crosslinking in this study are shown as solid circles. The arrangement of helices VII–XI has been described (reviewed in ref. 11). The placement of helix V has been established by site-directed chemical cleavage (23), site-directed electron spin resonance, and thiol crosslinking (24). The positioning of helices I and II is based on the results of this study.
