Figure 3.
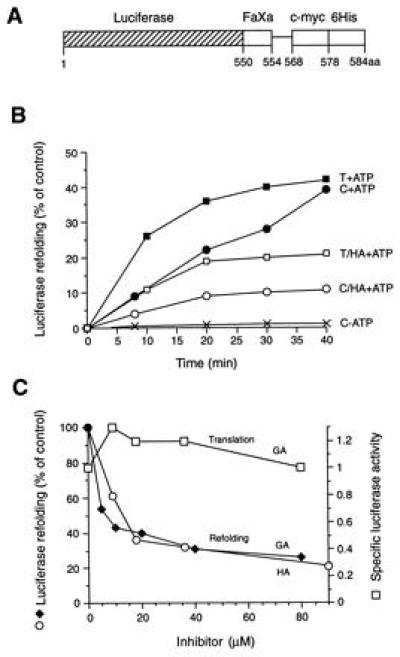
Refolding of firefly luciferase in RL. (A) Schematic representation of the luciferase-myc-His fusion construct used as a substrate (FaXa, factor Xa cleavage site; c-myc, c-myc epitope; 6His, 6Histidine-tag). The factor Xa cleavage site is separated from the c-myc epitope by a 14-amino acid spacer. (B) Refolding of thermally denatured (T) and chemically denatured (C) luciferase in control lysate (RL) in the presence and absence of ATP and in HA-treated lysate as indicated. The activity of an equivalent amount of native luciferase is set to 100%. (C) Effect of increasing concentrations of HA and GA on the refolding of chemically denatured luciferase (○, ♦) and on the de novo folding of newly translated luciferase (□). Enzyme activities were measured after 40 min of refolding or after 60 min of translation. Specific luciferase activities were calculated as the ratio of enzyme activity:full-length luciferase with the specific activity in untreated RL set to 1.0. Note that refolding was not inhibited when purified luciferase was incubated with HA or GA before or during chemical denaturation, and free drug was removed by gel filtration before refolding; inhibition persisted, however, when drug-treated RL was gel-filtered before the addition of unfolded luciferase (not shown). In contrast to Hsp90, luciferase itself did not bind to GA-agarose beads.
