Figure 6.
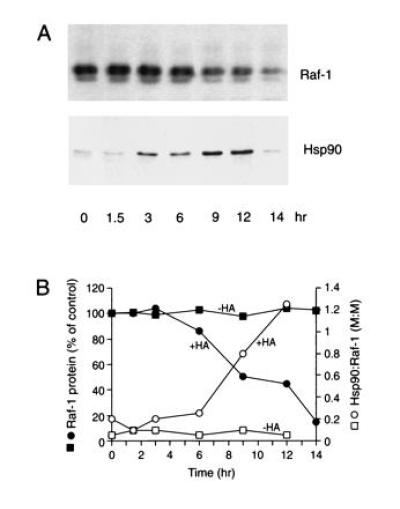
HA-induced degradation of Raf-1 in human breast carcinoma cells. (A) Raf-1 immunoprecipitates from MCF-7 cells, cultured at 37°C in the presence of HA, were analyzed by SDS/PAGE and immunoblotting with anti-Raf-1 and anti-Hsp90 antibodies. Raf-1 (74 kDa) migrates as a double-band due to phosphorylation. Direct immunoblotting of cells confirmed the degradation of essentially all Raf-1. (B) Time-dependent formation of Hsp90:Raf-1 complex and Raf-1 degradation. Depending on the efficiency of Hsp90 coimmunoprecipitation with anti-Raf-1 antibody (probably less than 100%), the results are consistent with a 1:1 or 1:2 stoichiometry of Raf-1:Hsp90 in the complex. Note that HA caused an approximately 2-fold increase in total Hsp90 (32) (not shown).
