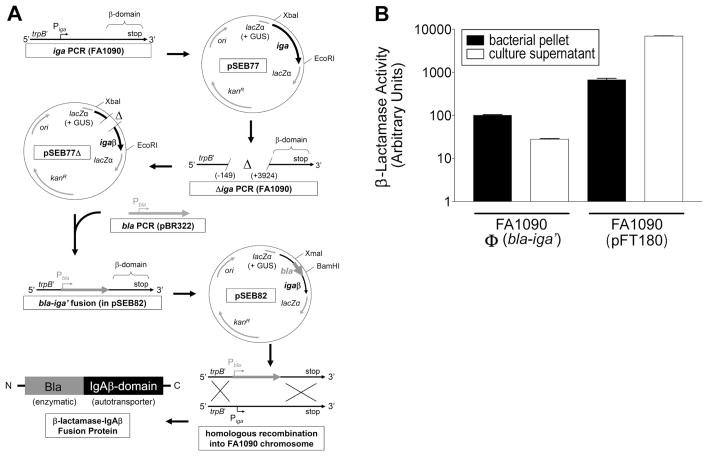
Figure 1.
GC strain FA1090Φ(bla-iga′) expresses the outer membrane anchored Bla-IgAβ fusion protein. (A) The cloning strategy to construct Bla-IgAβ is shown. The IgA protease gene (iga), was amplified by the PCR from FA1090 chromosomal DNA using primers iga-beta F2 and iga-beta R2. The iga PCR product was digested with EcoRI and XbaI and directionally cloned into pK18UP forming plasmid pSEB77 (GUS = gonococcal uptake sequence). The −149 to +3924 bp region of iga was deleted from pSEB77 utilizing deletion PCR with primers iga-beta del F and iga-beta del R. The deletion PCR product was digested with the BamHI and religated onto itself (pSEB77Δ). The β-lactamase (bla) gene (excluding the stop codon sequence) was amplified via the PCR from pBR322 using primers bla F and bla R, digested with BamH1 and XmaI, and directionally cloned into the deletion site of pSEB77Δ to generate the bla-iga′ gene fusion (pSEB82). Strain FA1090 was transformed with pSEB82. Transformants where the bla-iga′ construct replaced the iga gene in the FA1090 chromosome via homologous recombination [FA1090Φ(bla-iga′)] were selected on GCK Pen. (B) Pellets (black bars) or culture supernatants (white bars) of GC cultures were incubated with 50 μg/ml nitrocefin at room temperature for 30 min. Reactions were immediately centrifuged and the OD520 nm of the supernatants was measured. The Bla activity for the FA1090Φ(bla-iga′) pellet in a selected trial was defined as 100 aU and used as the standard to normalize the Bla activity of all other samples. The data are representative of three independent experiments performed in triplicate.