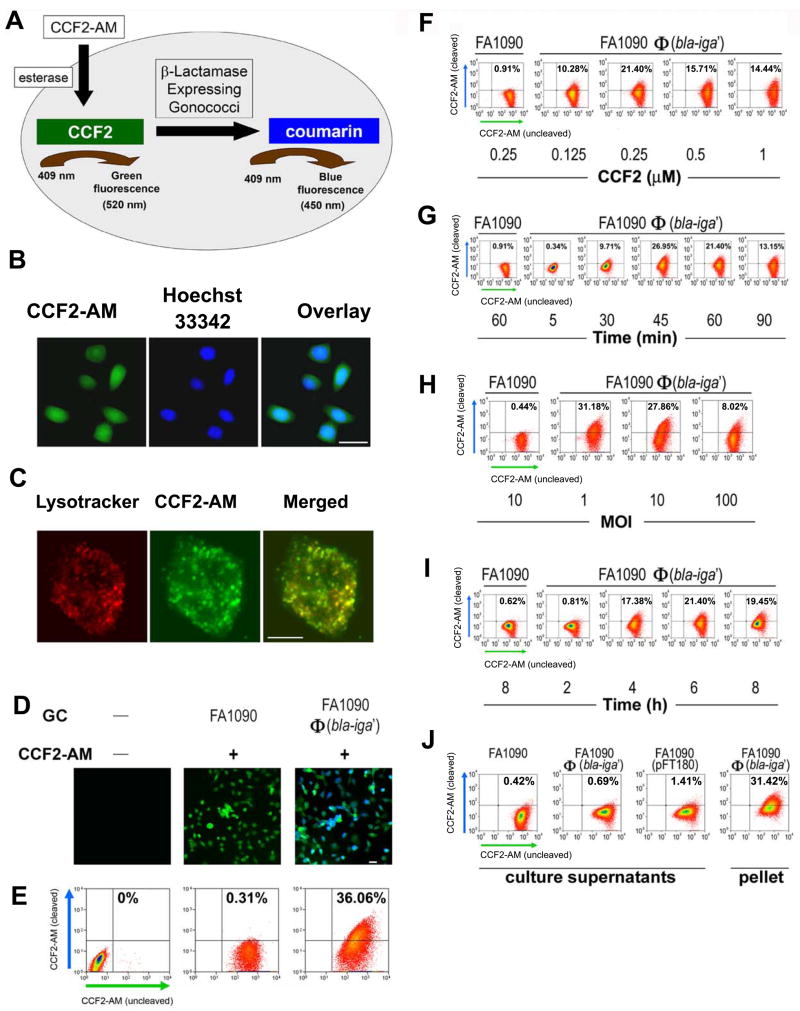
Figure 3.
Analyses of FA1090Φ(bla-iga′) internalization by ME180 cells utilizing the Bla reporter assay. (A) An overview of the internalization detection process in the Bla reporter assay. (B) ME180 cells were incubated simultaneously at room temperature with 1 μM CCF2-AM (green) for 90 min and Hoechst 33342 (blue) (100 ng/ml) for the final 60 min. Bar, 20 μm. (C) ME180 cells were incubated with 50 nM LysoTracker Red DND-99 for 30 min at 37°C and cells were shifted to room temperature where they were loaded with 1 μM CCF2-AM for an additional 90 min. Bar, 5 μm. (D) ME180 cells were incubated with or without GC for 6 h at 37°C, 5% CO2 and afterwards loaded with or without 1 μM CCF2-AM for 90 min. Bar, 20 μm. In (E–J), 1 × 106 ME180 cells/well were infected with FA1090Φ(bla-iga′) at an MOI 10 or culture supernatants derived from cultures containing 1 × 108 GC/ml (J) at 37°C, 5% CO2 for 6 h (unless otherwise indicated). For flow cytometry analysis (E–J), ME180 cells were then incubated with 0.25 μM CCF2-AM for 45–60 min. (F) Infected ME180 cells were loaded with different concentrations of CCF2-AM for 45–60 min. (G) Infected ME180 cells were loaded with CCF2-AM for different lengths of time. (H) ME180 cells were incubated with GC at different MOIs before being loaded with CCF2-AM. (I) ME180 cells were incubated with GC for different lengths of time before being stained with CCF2-AM. (J) ME180 cells were incubated with filtered supernatants (with varied levels of Bla activity) derived from overnight broth cultures of wild-type FA1090 (<1 aU), FA1090Φ(bla-iga′) (<3 aU), or FA1090(pFT180) (~700 aU) and then loaded with CCF2-AM. ME180 cells were incubated with FA1090Φ(bla-iga′) at an MOI 10 (10 aU) as a positive control for CCF2-AM cleavage in part H. In (E–J), ME180 cells incubated with FA1090 or FA1090 culture supernatant served as flow cytometry gating controls and all flow cytometry dot plots shown are representative data of two to five independent experiments. All GC used in these experiments were Pil+Opa+. Image quality was optimized using the AxioVision 4.6 software release from Carl Zeiss North America.