Figure 6.
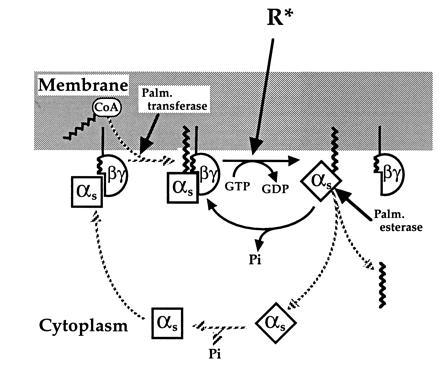
Model of the αs palmitoylation cycle. In the resting state, αs is held at the membrane by the hydrophobic interaction of its attached palmitate with the bilayer and by association with βγ, which is itself attached to the membrane. In addition, palmitoylation enhances affinity of αs for βγ and βγ reciprocally promotes the palmitoylated state by protecting αs from attack of palmitoyl esterase and by enhancing palmitoylation catalyzed by palmitoyl transferase. Receptor-triggered activation of αs induces its translocation to cytoplasm by causing subunit dissociation and rapid depalmitoylation. After converting bound GTP to GDP, cytosolic αs returns to the membrane by binding βγ, followed by repalmitoylation by the transferase. In comparison to our previous model (3), the proposed cycle stresses reciprocal regulation by palmitate and βγ and specifies the order of steps required for reattachment of cytosolic αs to the membrane.
