Abstract
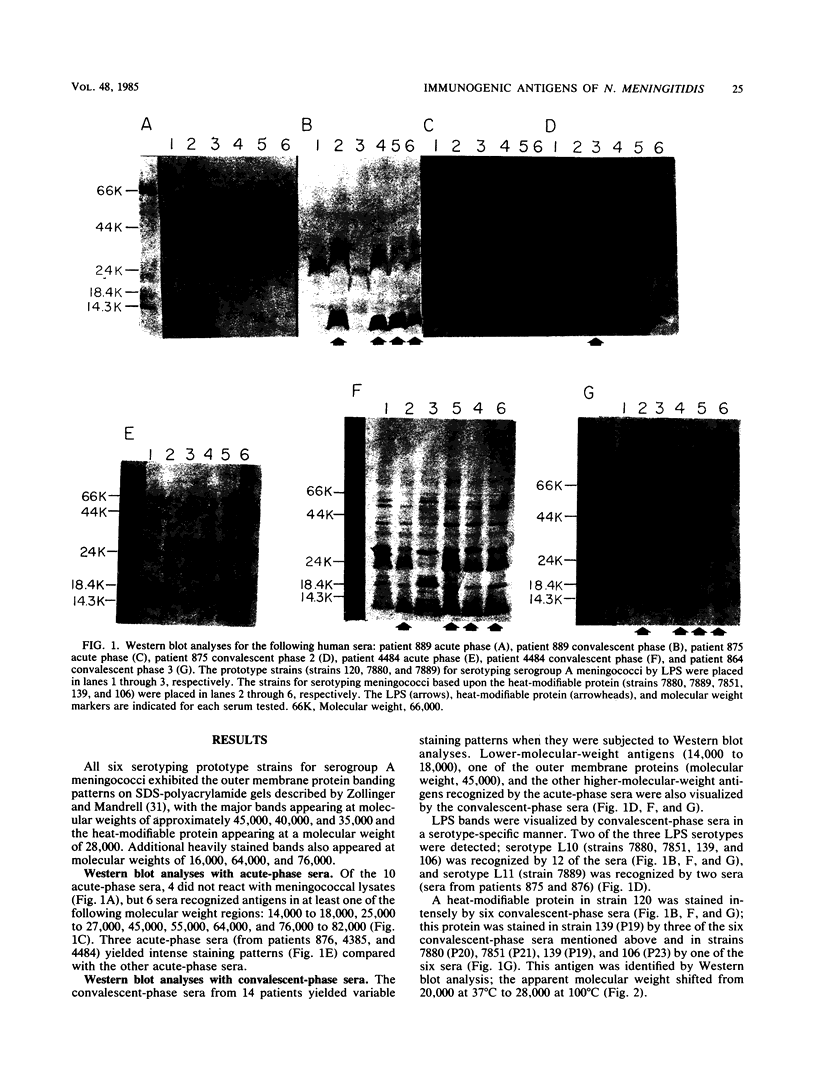
The antigens of Neisseria meningitidis serogroup A which were recognized by human antisera were identified by Western blot and enzyme-linked immunosorbent assay techniques. The components of six prototype strains used for serotyping serogroup A meningococci were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose for immunoperoxidase staining with sera collected from 10 acute-phase and 14 convalescent-phase patients. Six acute-phase sera detected six major antigens having apparent molecular weights between 14,000 and 82,000. In addition to recognizing these antigens, the convalescent-phase sera detected a protease-sensitive antigen with an apparent molecular weight of 20,000 for one strain and 27,000 for five strains, lipopolysaccharide, and the heat-modifiable proteins. The sera recognized lipopolysaccharide in a serotype-specific manner, whereas their reactions with the heat-modifiable protein were not serotype specific. Convalescent-phase sera recognized components from eight meningococcal serogroups. The concentrations of immunoglobulin G directed to capsular polysaccharide were determined by the enzyme-linked immunosorbent assay; seven acute-phase sera had less than 0.39 micrograms of antibody per ml, whereas the average concentration in convalescent-phase sera was 3.22 micrograms/ml and the range was 0.40 to 7.50 micrograms/ml.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt B. L., Artenstein M. S., Smith C. D. Antibody responses to meningococcal polysaccharide vaccines. Infect Immun. 1973 Oct;8(4):590–596. doi: 10.1128/iai.8.4.590-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Canellas P. F., Karu A. E. Statistical package for analysis of competition ELISA results. J Immunol Methods. 1981;47(3):375–385. doi: 10.1016/0022-1759(81)90294-5. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. F., Gotshlich E. C. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979 Nov;140(5):690–697. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Gotschlich E. C. Immune Response of human infants of polysaccharide vaccines of group A and C Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S31–S35. doi: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M. Epidemiological value of lipopolysaccharide and heat-modifiable outer-membrane protein serotyping of group-A strains of Neisseria meningitidis. J Med Microbiol. 1982 Aug;15(3):327–330. doi: 10.1099/00222615-15-3-327. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Brandt B. L., Artenstein M. S. Antigenic specificity of bactericidal antibodies in antisera to Neisseria meningitidis. J Infect Dis. 1973 Apr;127(4):378–387. doi: 10.1093/infdis/127.4.378. [DOI] [PubMed] [Google Scholar]
- Käyhty H. Comparison of passive hemagglutination, bactericidal activity, and radioimmunological methods in measuring antibody responses to Neisseria meningitidis group A capsular polysaccharide vaccine. J Clin Microbiol. 1980 Aug;12(2):256–263. doi: 10.1128/jcm.12.2.256-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Jousimies-Somer H., Peltola H., Mäketä P. H. Antibody response to capsular polysaccharides of groups A and C neisseria meningitidis and Haemophilus influenzae type b during bacteremic disease. J Infect Dis. 1981 Jan;143(1):32–41. doi: 10.1093/infdis/143.1.32. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Sarna S., Mäkelä P. H. Serum antibodies to capsular polysaccharide vaccine of group A Neissera meningitidis followed for three years in infants and children. J Infect Dis. 1980 Dec;142(6):861–868. doi: 10.1093/infdis/142.6.861. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Schneerson R., Sutton A. Class-specific antibody response to Haemophilus influenzae type b capsular polysaccharide vaccine. J Infect Dis. 1983 Oct;148(4):767–767. doi: 10.1093/infdis/148.4.767. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Peltola H. Group A meningococcal polysaccharide vaccine and course of the group A meningococcal epidemic in Finland. Scand J Infect Dis. 1978;10(1):41–44. doi: 10.3109/inf.1978.10.issue-1.09. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel J. E., Quan A. Homogeneity of protein serotype antigens in Neisseria meningitidis group A. Infect Immun. 1977 May;16(2):623–627. doi: 10.1128/iai.16.2.623-627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawara R. J., Prato C. M., Sippel J. E. Enzyme-linked immunosorbent assay with a monoclonal antibody for detecting group A meningococcal antigens in cerebrospinal fluid. J Clin Microbiol. 1984 Feb;19(2):230–234. doi: 10.1128/jcm.19.2.230-234.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Boslego J. W. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J Immunol Methods. 1981;46(2):129–140. doi: 10.1016/0022-1759(81)90130-7. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Pennington C. L., Artenstein M. S. Human antibody response to three meningococcal outer membrane antigens: comparison by specific hemagglutination assays. Infect Immun. 1974 Nov;10(5):975–984. doi: 10.1128/iai.10.5.975-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]