Figure 2.
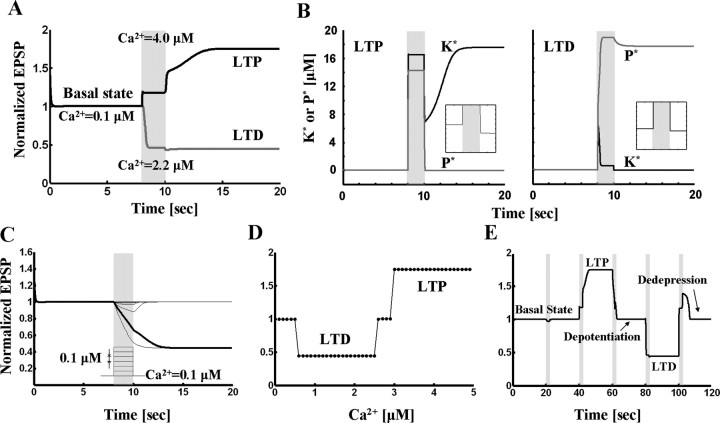
Coupled kinase and phosphatase switches can produce tristability. A, Simulation results for coupled switch model. The basal state undergoes LTP in response to high concentration (4 μm for 2 s) of Ca2+. The basal state goes to LTD state in response to moderate Ca2+ elevation (2.2 μm for 2 s). B, Enzyme activities during LTP and LTD. Left panel, Kinase and phosphatase activities during LTP. K* and P* denote the concentration of active kinase and phosphatase respectively. Before Ca2+ stimulation, both kinase and phosphatase are nearly inactive. During high level of Ca2+ application, kinase activity is dominant. After Ca2+ removal, active kinase represses the phosphatase activity below its initial level (see inset). Right panel, Kinase and phosphatase activities during LTD. Phosphatase is activated by Ca2+ pulse of 1 μm for 2 s and stays in the active state after Ca2+ is removed. During maintenance of LTD, active phosphatase represses kinase activity (see inset). C, Robustness of basal state to perturbation. Different levels of Ca2+ pulses (step size, 0.1 μm for 2 s) are applied on top of basal concentration (0.1 μm), and responses are traced. Basal state is robust to the Ca2+ concentration less than or equal to 0.5 μm. Concentrations >0.6 μm evoke LTD (red trace). D, Dependence of sign of synaptic modification on Ca2+ level during a pulse (note similarity to BCM curve). E, Reversals of LTP and LTD. The basal state which is robust to small perturbation (the first stimulation: 0.4 μm Ca2+ for 2 s) undergoes LTP by the second stimulation (4 μm for 2 s). The third stimulation (2.2 μm for 1.4 s) reverses the potentiated state back to basal level (depotentiation). The fourth (2.2 μm for 2 s) and the fifth (2.93 μm for 2 s) stimulations induce and reverse LTD, respectively (dedepression).