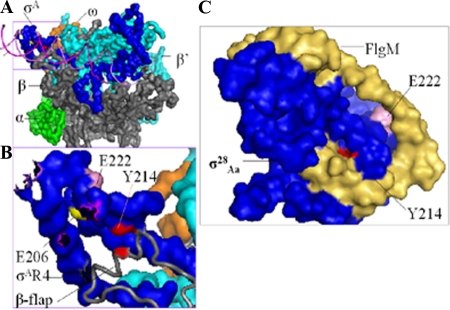
FIG. 7.
Positions of residues in substitutions that affect the interaction of σ28Ct region 4 with the β-flap. (A) Locations of residues based on the crystal structures of Thermus aquaticus σA holoenzyme and DNA (PDB ID no. 1I9Z) (32). Shown are the surfaces of α (green), β-flap (gray), β′ (cyan), ω (orange), and σ factor (blue). The DNA promoter (magenta) is shown as a cartoon. (B) Enlarged illustration of region 4 of T. aquaticus σA (labeled with corresponding amino acid numbers in σ28Ct) (see amino acid alignments in Fig. 2) and the β-flap contact. The β-flap (gray) is shown as the cartoon. The DNA promoter is hidden. (C) Locations of residues based on the crystal structures of the σ28Aa/FlgM complex (labeled with corresponding amino acid numbers in chlamydial σ28Ct) (PDB ID no. 1SC5 and 1RP3) (41). Blue, σ28Aa; yellow-brown, FlgM. Residues corresponding to the T aquaticus σA or σ28Aa were identified and mutated to the naturally occurring residues in chlamydial σ28Ct as indicated. This figure was generated using Pymol 0.99rc6 (http://pymol.sourceforge.net).