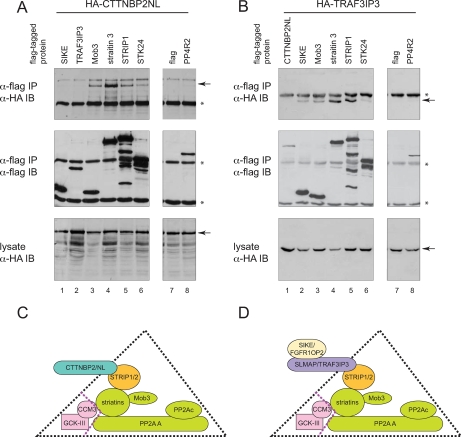
Fig. 4.
Mutually exclusive associations with STRIPAK. A and B, STRIPAK assembles with CTTNBP2NL or TRAF3IP3 in a mutually exclusive manner. Immunoprecipitation on anti-FLAG-Sepharose beads was performed on lysate from HEK293 cells transiently co-expressing the indicated FLAG- and HA-tagged constructs. To monitor specificity of the interactions, negative controls included FLAG alone as well as FLAG-PP4R2. Immune complexes were resolved by SDS-PAGE followed by transfer to nitrocellulose. Co-precipitation of HA-tagged proteins was detected by immunoblotting (IB) for the HA epitope (top panels; position of the tagged protein is indicated by an arrow. Asterisks indicate the heavy chains.). The precipitated FLAG-tagged protein was detected with anti-FLAG antibodies (middle panels). The expression of the HA-tagged protein in all samples is comparable (bottom panels). C and D, model for mutually exclusive interactions with STRIPAK. Our data suggest that STRIP proteins (orange) associate with the striatins, Mob3, and the phosphatases (green) in a stable complex. Associated with this complex are the CCM3 and GCK-III kinases (pink); these are likely to be more weakly associated as they were not recovered in TAPs. This core complex, STRIPAK, may associate with CTTNBP2 family proteins (C) or with a second complex containing SLMAP and SIKE family proteins (D).