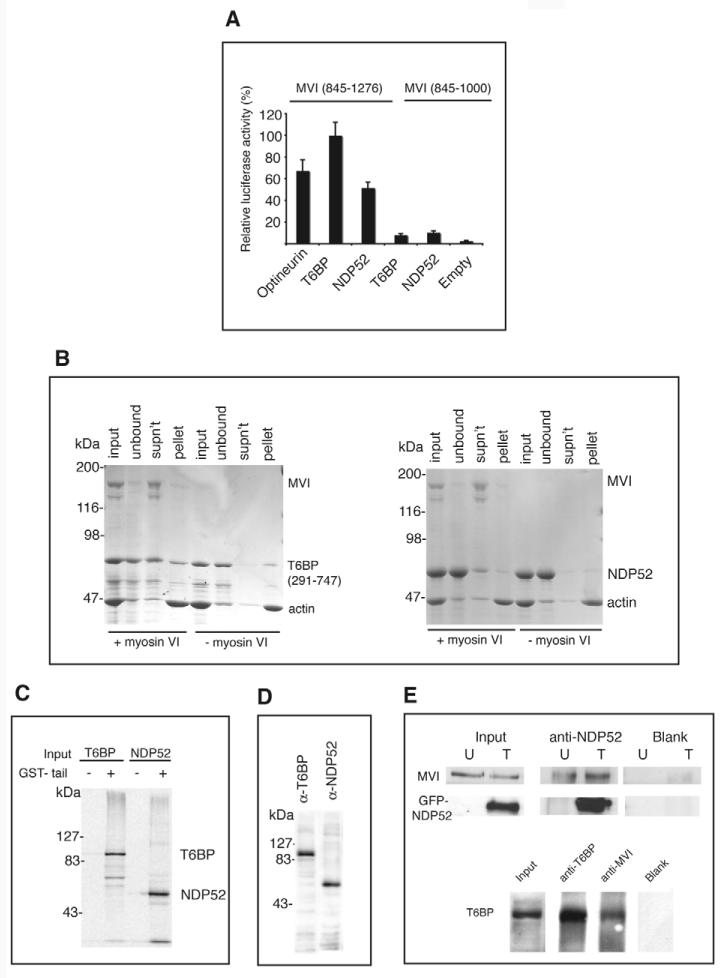
Fig. 1.
T6BP and NDP52 are myosin VI binding partners. (A) T6BP and NDP52 interact with the myosin VI C-terminal tail in the mammalian two-hybrid assay. Plasmids encoding myosin VI tail constructs were in the bait vector and T6BP or NDP52 were in the prey vector. (B) T6BP and NDP52 interact with myosin VI in an F-actin pelleting assay. Each binding partner was mixed with full-length myosin VI and then with F-actin (input). After centrifugation, to remove the unbound fraction (unbound), the actomyosin pellet was resuspended in salt-ATP solution to dissociate myosin VI from F-actin. A second centrifugation step partitioned myosin VI and T6BP/NDP52 into the supernatant (SN) and F-actin into the pellet (P). Control samples without myosin VI show that T6BP and NDP52 do not bind to F-actin. (C) NDP52 and full-length T6BP bind the myosin VI tail in a pull-down assay using GST-myosin VI tail and in-vitro-translated T6BP and NDP52. GST was used as a control. (D) HeLa cell lysates probed with our affinity-purified rabbit polyclonal antibodies raised against T6BP or NDP52. (E) T6BP or NDP52 co-immunoprecipitate with myosin VI. HeLa cells either untransfected (U) or overexpressing GFP-NDP52 (T) were lysed and immunoprecipitation was performed using no antibody (Blank) or anti-NDP52. Immunoblots were tested using anti-myosin VI or anti-NDP52 antibodies. A431 cells were used for the T6BP-myosin VI co-immunoprecipitation with antibodies shown. Immunoblots were stained with anti-T6BP antibodies.