Fig. 9.
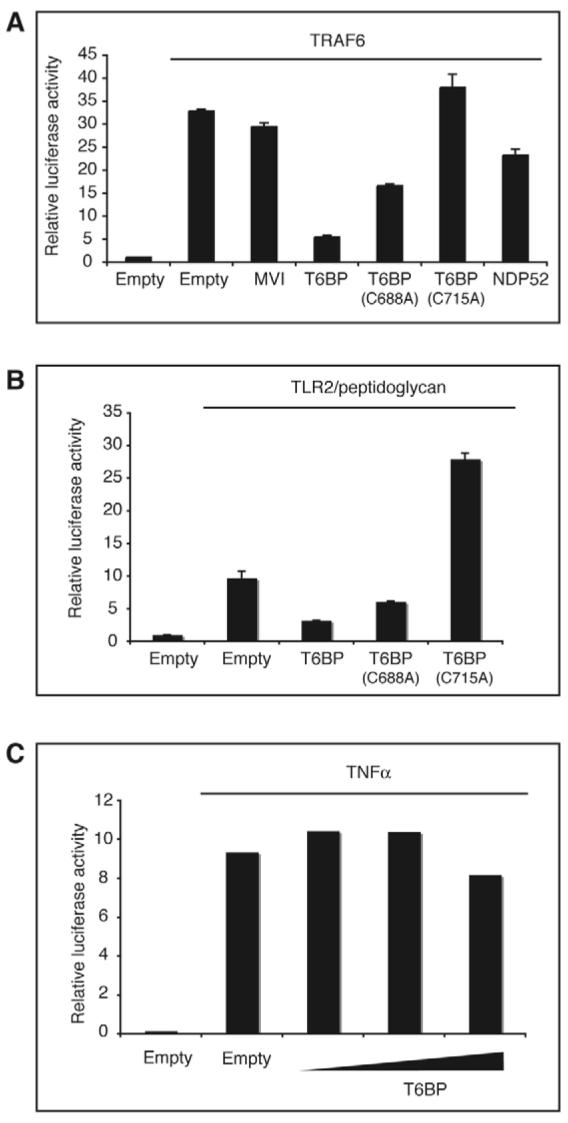
T6BP is an inhibitor of TRAF6-mediated NF-κB activation. (A) Overexpression of T6BP inhibits NF-κB activation induced by TRAF6. HEK293 ET cells were transfected with the plasmids encoding either GFP or wild-type or mutant T6BP, NDP52 or myosin VI (400 ng per well) and plasmids encoding TRAF6 (200 ng per well). An empty pEGFP vector was included as a control. At 24 hours after transfection the luciferase activities in cell lysates were measured. T6BP inhibits TRAF6-mediated NF-κB activation, whereas myosin VI and NDP52 have no significant effect. (B) Overexpression of T6BP inhibits NF-κB activation downstream of a TRAF6-dependent pathway. HEK293 ET cells were transfected with the plasmids encoding either GFP or wild-type or mutant T6BP (600 ng per well) and plasmids encoding TLR2 (100 ng per well). At 24 hours after transfection, cells were stimulated for 5 hours with 10 μg/ml of peptidoglycan. The luciferase activities in cell lysates were then measured. (C) NF-κB activation via a TRAF6-independent pathway is unaffected by T6BP overexpression. HEK293 ET cells were transfected with the GFP plasmid or increasing amounts (200, 400 and 800 ng per well) of T6BP plasmid. At 24 hours after transfection cells were stimulated for 5 hours with 10 ng/ml of TNF-α and then the luciferase activities in the cell lysates were measured.
