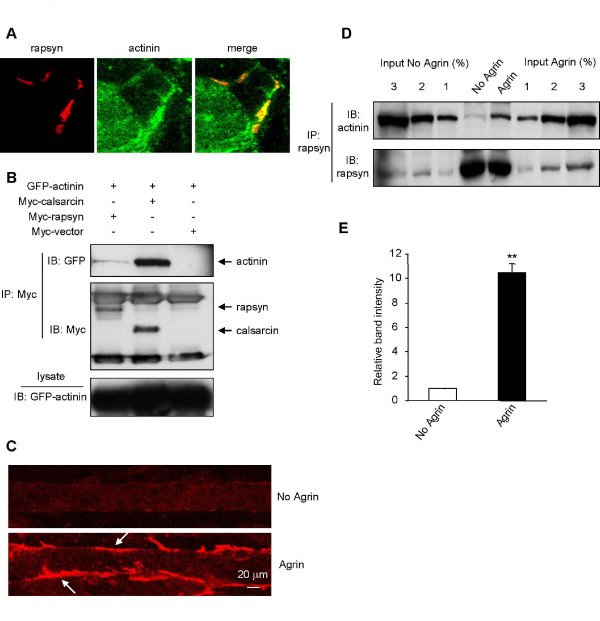
Figure 2.
Regulation of the rapsyn-α-actinin interaction by agrin. (A) Colocalization of α-actinin and rapsyn. Cross sections of adult mouse diaphragm were costained with antibodies specific for α-actinin and rapsyn. α-Actinin and rapsyn immunoreactivity was visualized by FITC and Cy3 conjugated secondary antibodies, respectively. (B) HEK 293 cells were cotransfected with GFP-α-actinin, Myc-calsarcin, Myc-rapsyn or Myc empty vector. Cell lysates were incubated with anti-Myc antibody followed by protein G agarose beads. Resulting precipitates were probed by indicated antibodies. (C) Ability of agrin to stimulate AChR clustering. C2C12 myotubes were stimulated without (control) or with 10 ng/ml agrin for 18 hr, and stained with Alexa 594-conjugated α-BTX. (D) Increased interaction in agrin-stimulated C2C12 myotubes. Lysates of control and stimulated myotubes were incubated with a rapsyn specific antibody followed by protein A agarose beads. After separation by SDS-PAGE and membrane transfer, immunoblotting was carried out with an antibody specific to α-actinin. Percentage of lysate inputs was immunoblotted to quantify the immunoprecipitation. (E) Quantitation of blot. The amount of α-actinin interacting with rapsyn is significantly greater upon agrin treatment. Data represent mean ± SEM of three experiments. **, p < 0.01, two-tailed Student's paired t test.